Palladium-catalyzed asymmetric [3+2] cycloaddition of trimethylenemethane with imines
- PMID: 17887679
- PMCID: PMC2532588
- DOI: 10.1021/ja0753389
Palladium-catalyzed asymmetric [3+2] cycloaddition of trimethylenemethane with imines
Abstract
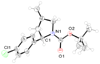
The transition metal catalyzed trimethylenemethane [3+2] cycloaddition provides a direct route to functionalized heterocycles. Herein, we describe a catalytic, asymmetric protocol for the reaction between 3-acetoxy-2-trimethylsilylmethyl-1-propene and various imines. The corresponding pyrrolidines were obtained in excellent yields and enantioselectivities making use of the novel phosphoramidite L10.
Figures
References
-
- O’Hagan D. Nat. Prod. Rep. 2000;17:435. - PubMed
-
- Harwood LM, Vickers RJ. In: Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. Padwa A, Pearson WH, editors. New York: John Wiley & Sons, Inc.; 2002. pp. 169–252.
-
- Yamago S. Organic Reactions. 2002;61:1.
-
- Jones MD, Kemmitt RDW. J. Chem. Soc., Chem. Commun. 1986:1201.
- Trost BM, Marrs CM. J. Am. Chem. Soc. 1993;115:6636.
- Yamago S, Nakamura M, Wang X-Q, Yanagawa M, Tokumitsu S, Nakamura E. J. Org. Chem. 1998;63:1694.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources