Sulfur K-edge X-ray absorption spectroscopy and density functional theory calculations on superoxide reductase: role of the axial thiolate in reactivity
- PMID: 17887751
- PMCID: PMC2533108
- DOI: 10.1021/ja064167p
Sulfur K-edge X-ray absorption spectroscopy and density functional theory calculations on superoxide reductase: role of the axial thiolate in reactivity
Abstract
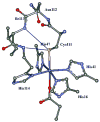
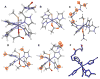
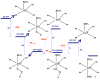
Superoxide reductase (SOR) is a non-heme iron enzyme that reduces superoxide to peroxide at a diffusion-controlled rate. Sulfur K-edge X-ray absorption spectroscopy (XAS) is used to investigate the ground-state electronic structure of the resting high-spin and CN- bound low-spin FeIII forms of the 1Fe SOR from Pyrococcus furiosus. A computational model with constrained imidazole rings (necessary for reproducing spin states), H-bonding interaction to the thiolate (necessary for reproducing Fe-S bond covalency of the high-spin and low-spin forms), and H-bonding to the exchangeable axial ligand (necessary to reproduce the ground state of the low-spin form) was developed and then used to investigate the enzymatic reaction mechanism. Reaction of the resting ferrous site with superoxide and protonation leading to a high-spin FeIII-OOH species and its subsequent protonation resulting in H2O2 release is calculated to be the most energetically favorable reaction pathway. Our results suggest that the thiolate acts as a covalent anionic ligand. Replacing the thiolate with a neutral noncovalent ligand makes protonation very endothermic and greatly raises the reduction potential. The covalent nature of the thiolate weakens the FeIII bond to the proximal oxygen of this hydroperoxo species, which raises its pKa by an additional 5 log units relative to the pKa of a primarily anionic ligand, facilitating its protonation. A comparison with cytochrome P450 indicates that the stronger equatorial ligand field from the porphyrin results in a low-spin FeIII-OOH species that would not be capable of efficient H2O2 release due to a spin-crossing barrier associated with formation of a high-spin 5C FeIII product. Additionally, the presence of the dianionic porphyrin pi ring in cytochrome P450 allows O-O heterolysis, forming an FeIV-oxo porphyrin radical species, which is calculated to be extremely unfavorable for the non-heme SOR ligand environment. Finally, the 5C FeIII site that results from the product release at the end of the O2- reduction cycle is calculated to be capable of reacting with a second O2-, resulting in superoxide dismutase (SOD) activity. However, in contrast to FeSOD, the 5C FeIII site of SOR, which is more positively charged, is calculated to have a high affinity for binding a sixth anionic ligand, which would inhibit its SOD activity.
Figures




References
-
- Jenney FE, Jr, Verhagen MFJM, Cui X, Adams MWW. Science. 1999;286:306–309. - PubMed
-
- Kurtz DM, Jr, Coulter ED. J Biol Inorg Chem. 2002;7:653–658. - PubMed
-
- Adams MWW, Jenney FE, Jr, Clay MD, Johnson MK. J Biol Inorg Chem. 2002;7:647–652. - PubMed
-
- Nivière V, Fontecave M. J Biol Inorg Chem. 2004;9:119–123. - PubMed
-
- Abreu IA, Xavier AV, LeGall J, Cabelli DE, Teixeira M. J Biol Inorg Chem. 2002;7:668–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

