Structural and thermodynamic consequences of 1-(4-chlorophenyl)imidazole binding to cytochrome P450 2B4
- PMID: 17887776
- PMCID: PMC2566820
- DOI: 10.1021/bi7011614
Structural and thermodynamic consequences of 1-(4-chlorophenyl)imidazole binding to cytochrome P450 2B4
Abstract
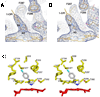
The crystal structure of P450 2B4 bound with 1-(4-chlorophenyl)imidazole (1-CPI) has been determined to delineate the structural basis for the observed differences in binding affinity and thermodynamics relative to 4-(4-chlorophenyl)imidazole (4-CPI). Compared with the previously reported 4-CPI complex, there is a shift in the 1-CPI complex of the protein backbone in helices F and I, repositioning the side chains of Phe-206, Phe-297, and Glu-301, and leading to significant reshaping of the active site. Phe-206 and Phe-297 exchange positions, with Phe-206 becoming a ligand-contact residue, while Glu-301, rather than hydrogen bonding to the ligand, flips away from the active site and interacts with His-172. As a result the active site volume expands from 200 A3 in the 4-CPI complex to 280 A3 in the 1-CPI complex. Based on the two structures, it was predicted that a Phe-206-->Ala substitution would alter 1-CPI but not 4-CPI binding. Isothermal titration calorimetry experiments indicated that this substitution had no effect on the thermodynamic signature of 4-CPI binding to 2B4. In contrast, relative to wild-type 1-CPI binding to F206A showed significantly less favorable entropy but more favorable enthalpy. This result is consistent with loss of the aromatic side chain and possible ordering of water molecules, now able to interact with Glu-301 and exposed residues in the I-helix. Hence, thermodynamic measurements support the active site rearrangement observed in the crystal structure of the 1-CPI complex and illustrate the malleability of the active site with the fine-tuning of residue orientations and thermodynamic signatures.
Figures


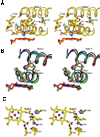

Similar articles
-
Conformational flexibility of mammalian cytochrome P450 2B4 in binding imidazole inhibitors with different ring chemistry and side chains. Solution thermodynamics and molecular modeling.J Biol Chem. 2006 Mar 24;281(12):8051-61. doi: 10.1074/jbc.M509696200. Epub 2006 Jan 26. J Biol Chem. 2006. PMID: 16439365
-
Structure of mammalian cytochrome P450 2B4 complexed with 4-(4-chlorophenyl)imidazole at 1.9-A resolution: insight into the range of P450 conformations and the coordination of redox partner binding.J Biol Chem. 2004 Jun 25;279(26):27294-301. doi: 10.1074/jbc.M403349200. Epub 2004 Apr 20. J Biol Chem. 2004. PMID: 15100217
-
Structure of microsomal cytochrome P450 2B4 complexed with the antifungal drug bifonazole: insight into P450 conformational plasticity and membrane interaction.J Biol Chem. 2006 Mar 3;281(9):5973-81. doi: 10.1074/jbc.M511464200. Epub 2005 Dec 21. J Biol Chem. 2006. PMID: 16373351
-
Thermodynamics of ligand binding to P450 2B4 and P450eryF studied by isothermal titration calorimetry.Drug Metab Rev. 2007;39(2-3):539-56. doi: 10.1080/03602530701498182. Drug Metab Rev. 2007. PMID: 17786637 Review.
-
Structure-function analysis of cytochromes P450 2B.Biochim Biophys Acta. 2007 Mar;1770(3):402-12. doi: 10.1016/j.bbagen.2006.07.006. Epub 2006 Jul 22. Biochim Biophys Acta. 2007. PMID: 16935426 Review.
Cited by
-
Carboetomidate: an analog of etomidate that interacts weakly with 11β-hydroxylase.Anesth Analg. 2013 Jun;116(6):1249-56. doi: 10.1213/ANE.0b013e31828b3637. Epub 2013 Mar 14. Anesth Analg. 2013. PMID: 23492967 Free PMC article.
-
Plasticity of CYP2B enzymes: structural and solution biophysical methods.Curr Drug Metab. 2012 Feb;13(2):167-76. doi: 10.2174/138920012798918417. Curr Drug Metab. 2012. PMID: 22208531 Free PMC article. Review.
-
Structure and function of cytochromes P450 2B: from mechanism-based inactivators to X-ray crystal structures and back.Drug Metab Dispos. 2011 Jul;39(7):1113-21. doi: 10.1124/dmd.111.039719. Epub 2011 Apr 18. Drug Metab Dispos. 2011. PMID: 21502194 Free PMC article.
-
Crystal structure of a cytochrome P450 2B6 genetic variant in complex with the inhibitor 4-(4-chlorophenyl)imidazole at 2.0-A resolution.Mol Pharmacol. 2010 Apr;77(4):529-38. doi: 10.1124/mol.109.062570. Epub 2010 Jan 8. Mol Pharmacol. 2010. PMID: 20061448 Free PMC article.
-
Structural diversity of eukaryotic membrane cytochrome p450s.J Biol Chem. 2013 Jun 14;288(24):17082-90. doi: 10.1074/jbc.R113.452805. Epub 2013 Apr 30. J Biol Chem. 2013. PMID: 23632020 Free PMC article. Review.
References
-
- Rendic S. Summary of information on human CYP enzymes: Human P450 metabolism data. Drug Metabolism Reviews. 2002;34:83–448. - PubMed
-
- Zhao Y, Halpert JR. Structure-function analysis of cytochromes P450 2B. Biochim. Biophys. Acta. 2007;1770:402–412. - PubMed
-
- Johnson EF, Stout CD. Structural diversity of human xenobiotic-metabolizing cytochrome P450 monooxygenases. Biochem. Biophys. Res. Commun. 2005;338:331–336. - PubMed
-
- Poulos TL. Structural and functional diversity in heme monooxygenases. Drug Metab. Dispos. 2005;33:10–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

