Nickel-dependent oligomerization of the alpha subunit of acetyl-coenzyme a synthase/carbon monoxide dehydrogenase
- PMID: 17887777
- PMCID: PMC2528952
- DOI: 10.1021/bi7014663
Nickel-dependent oligomerization of the alpha subunit of acetyl-coenzyme a synthase/carbon monoxide dehydrogenase
Abstract
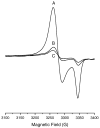
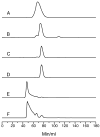
After activation with NiCl2, the recombinant alpha subunit of the Ni-containing alpha2beta2 acetyl-CoA synthase/carbon monoxide dehydrogenase (ACS/CODH) catalyzes the synthesis of acetyl-CoA from CO, CoA, and a methyl group donated from the corrinoid-iron-sulfur protein (CoFeSP). The alpha subunit has two conformations (open and closed), and contains a novel [Fe4S4]-[Nip Nid] active site in which the proximal Nip ion is labile. Prior to Ni activation, recombinant apo-alpha contain only an Fe4S4 cluster. Ni-activated alpha subunits exhibit catalytic, spectroscopic and heterogeneity properties typical of alpha subunits contained in ACS/CODH. Evidence presented here indicates that apo-alpha is a monomer whereas Ni-treated alpha oligomerizes, forming dimers and higher molecular weight species including tetramers. No oligomerization occurred when apo-alpha was treated with Cu(II), Zn(II), or Co(II) ions, but oligomerization occurred when apo-alpha was treated with Pt(II) and Pd(II) ions. The dimer accepted only 0.5 methyl group/alpha and exhibited, upon treatment with CO and under reducing conditions, the NiFeC EPR signal quantifying to 0.4 spin/alpha. Dimers appear to consist of two types of alpha subunits, including one responsible for catalytic activity and one that provides a structural scaffold. Higher molecular weight species may be similarly constituted. It is concluded that Ni binding to the A-cluster induces a conformational change in the alpha subunit, possibly to the open conformation, that promotes oligomerization. These interrelated events demonstrate previously unrealized connections between (a) the conformation of the alpha subunit; (b) the metal which occupies the proximal/distal sites of the A-cluster; and (c) catalytic activity.
Figures



References
-
- Lindahl PA, Graham DE. Acetyl-Coenzyme A synthases and Nickel-containing carbon monoxide dehydrogenases. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in Life Sciences. Vol. 2. John Wiley & Sons, Ltd.; Chichester, UK: 2007. pp. 357–416.
-
- Drennan CL, Doukov TI, Ragsdale SW. The metalloclusters of carbon monoxide dehydrogenase/acetyl-CoA synthase: a story in pictures. J Biol Inorg Chem. 2004;9:511–515. - PubMed
-
- Lindahl PA. Acetyl-Coenzyme A Synthase: The Case for a Nip0-Based Mechanism of Catalysis. J Biol Inorg Chem. 2004;9:516–524. - PubMed
-
- Volbeda A, Fontecilla-Camps JC. Crystallographic evidence for a CO/CO2 tunnel gating mechanism in the bifunctional carbon monoxide dehydrogenase/acetyl coenzyme A synthase from Moorella thermoacetica. J Biol Inorg Chem. 2004;9:525–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

