Therapeutic comparison of a new budesonide/formoterol pMDI with budesonide pMDI and budesonide/formoterol DPI in asthma
- PMID: 17887990
- PMCID: PMC2228375
- DOI: 10.1111/j.1742-1241.2007.01574.x
Therapeutic comparison of a new budesonide/formoterol pMDI with budesonide pMDI and budesonide/formoterol DPI in asthma
Abstract
Background: Budesonide/formoterol is an effective treatment for both asthma and chronic obstructive pulmonary disease. This study compared the efficacy and safety of a novel hydrofluoroalkane (HFA) pressurised metered-dose inhaler (pMDI) formulation of budesonide/formoterol with that of budesonide pMDI and budesonide/formoterol dry-powder inhaler (DPI; Turbuhaler).
Methods: This was a 12-week, multinational, randomised, double-blind, double-dummy study involving patients aged > or = 12 years with asthma. All patients had a forced expiratory volume in 1 s of 50-90% predicted normal and were inadequately controlled on inhaled corticosteroids (500-1600 microg/day) alone. Following a 2-week run-in, during which they received their usual medication, patients were randomised (two inhalations twice daily) to budesonide pMDI 200 microg, budesonide/formoterol DPI 160/4.5 microg or budesonide/formoterol pMDI 160/4.5 microg. The primary efficacy end-point was change from baseline in morning peak expiratory flow (PEF).
Results: In total, 680 patients were randomised, of whom 668 were included in the primary analysis. Therapeutically equivalent increases in morning PEF were observed with budesonide/formoterol pMDI (29.3 l/min) and budesonide/formoterol DPI (32.0 l/min) (95% confidence interval: -10.4 to 4.9; p = 0.48). The increase in morning PEF with budesonide/formoterol pMDI was significantly higher than with budesonide pMDI (+28.7 l/min; p < 0.001). Similar improvements with budesonide/formoterol pMDI vs. budesonide pMDI were seen for all secondary efficacy end-points. Both combination treatments were similarly well tolerated.
Conclusions: Budesonide/formoterol, administered via the HFA pMDI or DPI, is an effective and well-tolerated treatment for adult and adolescent patients with asthma, with both devices being therapeutically equivalent.
Figures



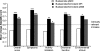
References
-
- National Institutes for Health. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. GINA Workshop Report. Bethesda, MD: National Institutes for Health, NIH Publication No. 02-3659; 1995. (updated 2004)
-
- Buhl R, Creemers JP, Vondra V, et al. Once-daily budesonide/formoterol in a single inhaler in adults with moderate persistent asthma. Respir Med. 2003;97:323–30. - PubMed
-
- Stallberg B, Olsson P, Jorgensen LA, et al. Budesonide/formoterol adjustable maintenance dosing reduces asthma exacerbations versus fixed dosing. Int J Clin Pract. 2003;57:656–61. - PubMed
-
- Fitzgerald JM, Sears MR, Boulet LP, et al. Adjustable maintenance dosing with budesonide/formoterol reduces asthma exacerbations compared with traditional fixed dosing: a five-month multicentre Canadian study. Can Respir J. 2003;10:427–34. - PubMed
-
- O'Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–36. - PubMed

