The TATA binding protein associated factor 4b (TAF4b) mediates FSH stimulation of the IGFBP-3 promoter in cultured porcine ovarian granulosa cells
- PMID: 17888567
- PMCID: PMC2211527
- DOI: 10.1016/j.mce.2007.08.004
The TATA binding protein associated factor 4b (TAF4b) mediates FSH stimulation of the IGFBP-3 promoter in cultured porcine ovarian granulosa cells
Abstract
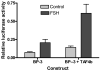
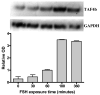
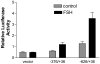
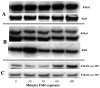
We have established the gene for IGF binding protein-3 (IGFBP-3) as a target for FSH action. FSH effects on this gene require the PKA pathway as well as the PI-3 kinase and MAPK pathways. At the IGFBP-3 promoter, FSH effects depend on a site for TATA box binding protein (TBP) and formation of a high molecular weight transcription complex. To further elucidate FSH effects on the downstream events involving the TBP site, we cloned a pig TAF4b cDNA into a P-Flag expression vector. By co-transfecting granulosa cells with the IGFBP-3 promoter, we found that TAF4b mimics and enhances FSH induction of IGFBP-3 reporter activity. Using RT-PCR we showed that FSH stimulates expression of TAF4b. This would suggest that the role of TAF4b in follicular development is regulated by FSH. TAF4b may thus be the TFIID component that binds to the TBP site on the IGFBP-3 promoter and is essential for FSH induction of IGFBP-3.
Figures

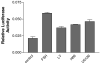
Similar articles
-
Follicle-stimulating hormone induction of ovarian insulin-like growth factor-binding protein-3 transcription requires a TATA box-binding protein and the protein kinase A and phosphatidylinositol-3 kinase pathways.Mol Endocrinol. 2005 Jul;19(7):1837-48. doi: 10.1210/me.2004-0487. Epub 2005 Feb 17. Mol Endocrinol. 2005. PMID: 15718291
-
Insulin-like growth factor-binding protein-3 in porcine ovarian granulosa cells: gene cloning, promoter mapping, and follicle-stimulating hormone regulation.Endocrinology. 2004 Apr;145(4):1776-85. doi: 10.1210/en.2003-1552. Epub 2004 Jan 8. Endocrinology. 2004. PMID: 14715717
-
Mechanisms of insulin-like growth factor I augmentation of follicle-stimulating hormone-induced porcine steroidogenic acute regulatory protein gene promoter activity in granulosa cells.Endocrinology. 1999 Jan;140(1):146-53. doi: 10.1210/endo.140.1.6407. Endocrinology. 1999. PMID: 9886819
-
Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells.Mol Endocrinol. 2000 Aug;14(8):1283-300. doi: 10.1210/mend.14.8.0500. Mol Endocrinol. 2000. PMID: 10935551
-
FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A.Cell Signal. 2006 Sep;18(9):1351-9. doi: 10.1016/j.cellsig.2006.02.011. Epub 2006 Apr 17. Cell Signal. 2006. PMID: 16616457 Free PMC article. Review.
Cited by
-
Overview of follicle stimulating hormone and its receptors in reproduction and in stem cells and cancer stem cells.Int J Biol Sci. 2022 Jan 1;18(2):675-692. doi: 10.7150/ijbs.63721. eCollection 2022. Int J Biol Sci. 2022. PMID: 35002517 Free PMC article. Review.
-
The mammalian ovary from genesis to revelation.Endocr Rev. 2009 Oct;30(6):624-712. doi: 10.1210/er.2009-0012. Epub 2009 Sep 23. Endocr Rev. 2009. PMID: 19776209 Free PMC article. Review.
-
Mapping the follicle-stimulating hormone-induced signaling networks.Front Endocrinol (Lausanne). 2011 Oct 5;2:45. doi: 10.3389/fendo.2011.00045. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22666216 Free PMC article.
-
Mechanisms controlling germline cyst breakdown and primordial follicle formation.Cell Mol Life Sci. 2017 Jul;74(14):2547-2566. doi: 10.1007/s00018-017-2480-6. Epub 2017 Feb 14. Cell Mol Life Sci. 2017. PMID: 28197668 Free PMC article. Review.
-
Circular RNA TAF4B targeting MFN2 accelerates cell growth in bladder cancer through p27 depression and AKT activation.Front Immunol. 2024 Oct 4;15:1477196. doi: 10.3389/fimmu.2024.1477196. eCollection 2024. Front Immunol. 2024. PMID: 39430741 Free PMC article.
References
-
- BIGGS JR, AHN NG, KRAFT AS. Activation of the mitogen-activated protein kinase pathway in U937 leukemic cells induces phosphorylation of the amino terminus of the TATA-binding protein. Cell Growth Differ. 1998;9:667–76. - PubMed
-
- BURGER AM, LEYLAND-JONES B, BANERJEE K, SPYROPOULOS DD, SETH AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer. 2005;41:1515–27. - PubMed
-
- COTTOM J, SALVADOR LM, MAIZELS ET, REIERSTAD S, PARK Y, CARR DW, DAVARE MA, HELL JW, PALMER SS, DENT P, KAWAKATSU H, OGATA M, HUNZICKER-DUNN M. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem. 2003;278:7167–79. - PMC - PubMed
-
- CUNNINGHAM MA, ZHU Q, UNTERMAN TG, HAMMOND JM. Follicle-stimulating hormone promotes nuclear exclusion of the forkhead transcription factor FoxO1a via phosphatidylinositol 3-kinase in porcine granulosa cells. Endocrinology. 2003;144:5585–94. - PubMed
-
- DIKSTEIN R, ZHOU S, TJIAN R. Human TAFII 105 is a cell type-specific TFIID subunit related to hTAFII130. Cell. 1996;87:137–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

