Innate and adaptive immune response to apoptotic cells
- PMID: 17888627
- PMCID: PMC2100400
- DOI: 10.1016/j.jaut.2007.07.017
Innate and adaptive immune response to apoptotic cells
Abstract
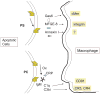
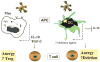
The immune system is constantly exposed to dying cells, most of which arise during central tolerance and from effete circulating immune cells. Under homeostatic conditions, phagocytes (predominantly macrophages and dendritic cells) belonging to the innate immune system, rapidly ingest cells and their debris. Apoptotic cell removal requires recognition of altered self on the apoptotic membrane, a process which is facilitated by natural antibodies and serum opsonins. Recognition, may be site and context specific. Uptake and ingestion of apoptotic cells promotes an immunosuppressive environment that avoids inflammatory responses to self-antigens. However, it does not preclude a T cell response and it is likely that constant exposure to self-antigen, particularly by immature dendritic cells, leads to T cell tolerance. Tolerance occurs by several different mechanisms including anergy and deletion (for CD8+T cells) and induction of T regulatory cells (for CD4+T cells). Failed apoptotic cell clearance promotes immune responses to self-antigens, especially when the cellular contents are leaked from the cell (necrosis). Inflammatory responses may be induced by nucleic acid stimulation of Toll like receptors and other immune sensors, specific intracellular proteins and non-protein (uric acid) stimulation of inflammasomes.
Figures

References
-
- Blank M, Shoenfeld Y. B cell targeted therapy in autoimmunity. J Autoimmun. 2007;28:62–8. - PubMed
-
- O’Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, Bush JA, Li G, Finegood DT, Dutz JP. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. 2006;26:104–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

