Regulatory T cells in the prevention of mucosal inflammatory diseases: patrolling the border
- PMID: 17889505
- PMCID: PMC2692919
- DOI: 10.1016/j.jaut.2007.07.021
Regulatory T cells in the prevention of mucosal inflammatory diseases: patrolling the border
Abstract
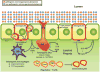
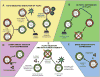
Regulatory T (Treg) cells are important contributors to the maintenance of immune tolerance in the periphery, and deficiency of Tregs is associated with various immunopathic diseases. Murine models of autoimmune and autoinflammatory disorders have helped to elucidate how Tregs are involved in these diseases. A feature in common between human and mice that lack one or another of the key Treg subsets is the occurrence of mucosal inflammation. The relatively fragile mucosal surface represents a complex system that is normally well equipped to ward off harmful pathogens yet at the same time is inhibitory to destructive inflammatory responses to biologically needed (probiotic) microorganisms, or other common environmental antigens e.g. nutrients. We here discuss the importance of Tregs in maintaining tolerance at mucosal surfaces and the outcomes of deficiency of Treg function. The intestinal tract and its inflammatory diseases provide the "point of departure" for discussion, but similar considerations could apply to other mucosal linings exposed to the environment such as other members of the digestive system. However, the lungs, bile ducts, urogenital tract and other mucosal surfaces are susceptible to poorly understood inflammatory states that possibly depend on dysfunction of Treg cells. Finally there are now potential therapies predicated on reconstitution of effective function of Treg cells.
Figures
Similar articles
-
Special regulatory T-cell review: Regulatory T cells and the intestinal tract--patrolling the frontier.Immunology. 2008 Jan;123(1):6-10. doi: 10.1111/j.1365-2567.2007.02778.x. Immunology. 2008. PMID: 18154611 Free PMC article. Review.
-
CD4+CD25+ T regulatory cells and TGF-beta in mucosal immune system: the good and the bad.Curr Med Chem. 2007;14(21):2245-9. doi: 10.2174/092986707781696591. Curr Med Chem. 2007. PMID: 17896973 Review.
-
Oral tolerance.Immunol Rev. 2011 May;241(1):241-59. doi: 10.1111/j.1600-065X.2011.01017.x. Immunol Rev. 2011. PMID: 21488901 Free PMC article. Review.
-
Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases.Immunol Lett. 2004 May 15;93(2-3):97-108. doi: 10.1016/j.imlet.2004.02.005. Immunol Lett. 2004. PMID: 15158604 Review.
-
Intestinal Regulatory T Cells.Adv Exp Med Biol. 2021;1278:141-190. doi: 10.1007/978-981-15-6407-9_9. Adv Exp Med Biol. 2021. PMID: 33523448 Free PMC article.
Cited by
-
The geo-epidemiology of temporal (giant cell) arteritis.Clin Rev Allergy Immunol. 2008 Oct;35(1-2):88-95. doi: 10.1007/s12016-008-8075-0. Clin Rev Allergy Immunol. 2008. PMID: 18286386 Review.
-
Repertoire-dependent immunopathology.J Autoimmun. 2007 Dec;29(4):257-61. doi: 10.1016/j.jaut.2007.07.019. Epub 2007 Sep 21. J Autoimmun. 2007. PMID: 17889507 Free PMC article.
-
Effector and suppressor T cells in celiac disease.World J Gastroenterol. 2015 Jun 28;21(24):7349-56. doi: 10.3748/wjg.v21.i24.7349. World J Gastroenterol. 2015. PMID: 26139981 Free PMC article. Review.
-
Etiopathogenesis of primary biliary cirrhosis.World J Gastroenterol. 2008 Jun 7;14(21):3328-37. doi: 10.3748/wjg.14.3328. World J Gastroenterol. 2008. PMID: 18528930 Free PMC article. Review.
-
Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization.Clin Exp Immunol. 2009 Mar;155(3):577-86. doi: 10.1111/j.1365-2249.2008.03837.x. Epub 2008 Dec 15. Clin Exp Immunol. 2009. PMID: 19094117 Free PMC article.
References
-
- Kunisawa J, Takahashi I, Kiyono H. Intraepithelial lymphocytes: their shared and divergent immunological behaviors in the small and large intestine. Immunol Rev. 2007;215:136–53. - PubMed
-
- Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4(12):953–64. - PubMed
-
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2(4):361–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

