Autoreactive B-1 B cells: constraints on natural autoantibody B cell antigen receptors
- PMID: 17889506
- PMCID: PMC2096705
- DOI: 10.1016/j.jaut.2007.07.020
Autoreactive B-1 B cells: constraints on natural autoantibody B cell antigen receptors
Abstract
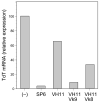
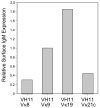
B-1 B-cells constitute a distinctive population of cells that are enriched for self-reactive B cell receptors (BCRs). These BCRs are encoded by a restricted set of heavy and light chains, including heavy chains that lack nontemplated nucleotide additions at the V-D and D-J joining regions. One prototype natural autoantibody produced by B-1 B cells binds to a cryptic determinant exposed on senescent red blood cells that includes the phosphatidylcholine (PtC) moiety. The V(H)11Vkappa9 BCR, which accounts for a large fraction of the anti-PtC specificity, is underrepresented in other B-cell populations, including newly formed B cells in bone marrow, and the transitional B cells, follicular B cells, and marginal zone B cells in spleen. Previous work has shown that V(H)11 heavy chains pair ineffectively with surrogate light chain (SLC) and so do not promote development in bone marrow, but instead allow fetal liver maturation because of a fetal preference for weaker pre-BCR signaling. Such inefficient SLC pairing constitutes one constraint on the maturation of B cells containing V(H)11 rearrangements that biases their generation to fetal development. Here, we examine another possible bottleneck to the B1 cell repertoire: light chain pairing with V(H)11 heavy chain, finding very significant preferences.
Figures



Similar articles
-
The skewed heavy-chain repertoire in peritoneal B-1 cells is predetermined by the selection via pre-B cell receptor during B cell ontogeny in the fetal liver.Int Immunol. 2009 Jan;21(1):43-52. doi: 10.1093/intimm/dxn122. Epub 2008 Nov 14. Int Immunol. 2009. PMID: 19011159
-
Selection during development of VH11+ B cells: a model for natural autoantibody-producing CD5+ B cells.Immunol Rev. 2004 Feb;197:60-74. doi: 10.1111/j.0105-2896.2004.0100.x. Immunol Rev. 2004. PMID: 14962187 Review.
-
Censoring of autoreactive B cell development by the pre-B cell receptor.Science. 2008 Aug 1;321(5889):696-9. doi: 10.1126/science.1157533. Epub 2008 Jun 19. Science. 2008. PMID: 18566249
-
Distorted antibody repertoire developed in the absence of pre-B cell receptor formation.Biochem Biophys Res Commun. 2018 Jan 1;495(1):1411-1417. doi: 10.1016/j.bbrc.2017.11.171. Epub 2017 Nov 27. Biochem Biophys Res Commun. 2018. PMID: 29191653
-
Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells.Immunol Rev. 2000 Jun;175:33-46. Immunol Rev. 2000. PMID: 10933589 Review.
Cited by
-
B cell subsets in atherosclerosis.Front Immunol. 2012 Dec 11;3:373. doi: 10.3389/fimmu.2012.00373. eCollection 2012. Front Immunol. 2012. PMID: 23248624 Free PMC article.
-
Etiopathogenesis of primary biliary cirrhosis.World J Gastroenterol. 2008 Jun 7;14(21):3328-37. doi: 10.3748/wjg.14.3328. World J Gastroenterol. 2008. PMID: 18528930 Free PMC article. Review.
-
The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease.Curr Opin Immunol. 2011 Jun;23(3):310-8. doi: 10.1016/j.coi.2011.03.003. Epub 2011 Apr 7. Curr Opin Immunol. 2011. PMID: 21482089 Free PMC article. Review.
-
Atherosclerosis and autoimmunity.Clin Rev Allergy Immunol. 2009 Aug;37(1):1-3. doi: 10.1007/s12016-008-8092-z. Clin Rev Allergy Immunol. 2009. PMID: 18985285 Review. No abstract available.
-
B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice.Hepatology. 2009 Dec;50(6):1893-903. doi: 10.1002/hep.23238. Hepatology. 2009. PMID: 19877182 Free PMC article.
References
-
- Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Advances in Immunology. 1994;55:297–339. - PubMed
-
- Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. - PubMed
-
- Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

