Experimental Rhodococcus equi and equine infectious anemia virus DNA vaccination in adult and neonatal horses: effect of IL-12, dose, and route
- PMID: 17889970
- PMCID: PMC3342688
- DOI: 10.1016/j.vaccine.2007.07.055
Experimental Rhodococcus equi and equine infectious anemia virus DNA vaccination in adult and neonatal horses: effect of IL-12, dose, and route
Abstract
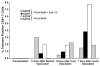
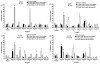
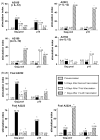
Improving the ability of DNA-based vaccines to induce potent Type1/Th1 responses against intracellular pathogens in large outbred species is essential. Rhodoccocus equi and equine infectious anemia virus (EIAV) are two naturally occurring equine pathogens that also serve as important large animal models of neonatal immunity and lentiviral immune control. Neonates present a unique challenge for immunization due to their diminished immunologic capabilities and apparent Th2 bias. In an effort to augment R. equi- and EIAV-specific Th1 responses induced by DNA vaccination, we hypothesized that a dual promoter plasmid encoding recombinant equine IL-12 (rEqIL-12) would function as a molecular adjuvant. In adult horses, DNA vaccines induced R. equi- and EIAV-specific antibody and lymphoproliferative responses, and EIAV-specific CTL and tetramer-positive CD8+ T lymphocytes. These responses were not enhanced by the rEqIL-12 plasmid. In neonatal foals, DNA immunization induced EIAV-specific antibody and lymphoproliferative responses, but not CTL. The R. equi vapA vaccine was poorly immunogenic in foals even when co-administered with the IL-12 plasmid. It was concluded that DNA immunization was capable of inducing Th1 responses in horses; dose and route were significant variables, but rEqIL-12 was not an effective molecular adjuvant. Additional work is needed to optimize DNA vaccine-induced Th1 responses in horses, especially in neonates.
Figures

Similar articles
-
Viral load and clinical disease enhancement associated with a lentivirus cytotoxic T lymphocyte vaccine regimen.Vaccine. 2009 Apr 21;27(18):2453-68. doi: 10.1016/j.vaccine.2009.02.048. Epub 2009 Feb 24. Vaccine. 2009. PMID: 19368787 Free PMC article.
-
Analysis of anamnestic immune responses in adult horses and priming in neonates induced by a DNA vaccine expressing the vapA gene of Rhodococcus equi.Vaccine. 2003 Sep 8;21(25-26):3815-25. doi: 10.1016/s0264-410x(03)00329-3. Vaccine. 2003. PMID: 12922115
-
Assessment in mice of vapA-DNA vaccination against Rhodococcus equi infection.Vet Immunol Immunopathol. 2005 Apr 8;104(3-4):215-25. doi: 10.1016/j.vetimm.2004.12.006. Vet Immunol Immunopathol. 2005. PMID: 15734542
-
Cytotoxic T lymphocytes in protection against equine infectious anemia virus.Anim Health Res Rev. 2004 Dec;5(2):271-6. doi: 10.1079/ahr200482. Anim Health Res Rev. 2004. PMID: 15984338 Review.
-
Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens during prime-booster vaccination regimens.Histol Histopathol. 2001 Apr;16(2):655-67. doi: 10.14670/HH-16.655. Histol Histopathol. 2001. PMID: 11332721 Review.
Cited by
-
Antibody activities in hyperimmune plasma against the Rhodococcus equi virulence -associated protein A or poly-N-acetyl glucosamine are associated with protection of foals against rhodococcal pneumonia.PLoS One. 2021 Aug 26;16(8):e0250133. doi: 10.1371/journal.pone.0250133. eCollection 2021. PLoS One. 2021. PMID: 34437551 Free PMC article.
-
Porcine IL-12 plasmid as an adjuvant improves the cellular and humoral immune responses of DNA vaccine targeting transmissible gastroenteritis virus spike gene in a mouse model.J Vet Med Sci. 2019 Oct 18;81(10):1438-1444. doi: 10.1292/jvms.18-0682. Epub 2019 Sep 2. J Vet Med Sci. 2019. PMID: 31474664 Free PMC article.
-
Oral Administration of Electron-Beam Inactivated Rhodococcus equi Failed to Protect Foals against Intrabronchial Infection with Live, Virulent R. equi.PLoS One. 2016 Feb 1;11(2):e0148111. doi: 10.1371/journal.pone.0148111. eCollection 2016. PLoS One. 2016. PMID: 26828865 Free PMC article.
-
Randomized, controlled trial comparing Rhodococcus equi and poly-N-acetyl glucosamine hyperimmune plasma to prevent R equi pneumonia in foals.J Vet Intern Med. 2021 Nov;35(6):2912-2919. doi: 10.1111/jvim.16294. Epub 2021 Nov 5. J Vet Intern Med. 2021. PMID: 34738651 Free PMC article.
-
Viral load and clinical disease enhancement associated with a lentivirus cytotoxic T lymphocyte vaccine regimen.Vaccine. 2009 Apr 21;27(18):2453-68. doi: 10.1016/j.vaccine.2009.02.048. Epub 2009 Feb 24. Vaccine. 2009. PMID: 19368787 Free PMC article.
References
-
- Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–74. - PubMed
-
- Hokey DA, Weiner DB. DNA vaccines for HIV: challenges and opportunities. Springer Semin Immunopathol. 2006;28(3):267–79. - PubMed
-
- Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75(9):4040–7. - PMC - PubMed
-
- Lunn DP, Soboll G, Schram BR, Quass J, McGregor MW, Drape RJ, et al. Antibody responses to DNA vaccination of horses using the influenza virus hemagglutinin gene. Vaccine. 1999;17(18):2245–58. - PubMed
-
- Soboll G, Nelson KM, Leuthner ES, Clark RJ, Drape R, Macklin MD, et al. Mucosal co-administration of cholera toxin and influenza virus hemagglutinin-DNA in ponies generates a local IgA response. Vaccine. 2003;21(21–22):3081–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

