Long-range nonanomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane
- PMID: 17890385
- PMCID: PMC2157255
- DOI: 10.1529/biophysj.107.115121
Long-range nonanomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane
Abstract
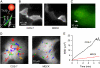
Aquaporin-1 (AQP1) is an integral membrane protein that facilitates osmotic water transport across cell plasma membranes in epithelia and endothelia. AQP1 has no known specific interactions with cytoplasmic or membrane proteins, but its recovery in a detergent-insoluble membrane fraction has suggested possible raft association. We tracked the membrane diffusion of AQP1 molecules labeled with quantum dots at an engineered external epitope at frame rates up to 91 Hz and over times up to 6 min. In transfected COS-7 cells, >75% of AQP1 molecules diffused freely over approximately 7 mum in 5 min, with diffusion coefficient, D(1-3) approximately 9 x 10(-10) cm(2)/s. In MDCK cells, approximately 60% of AQP1 diffused freely, with D(1-3) approximately 3 x 10(-10) cm(2)/s. The determinants of AQP1 diffusion were investigated by measurements of AQP1 diffusion following skeletal disruption (latrunculin B), lipid/raft perturbations (cyclodextrin and sphingomyelinase), and bleb formation. We found that cytoskeletal disruption had no effect on AQP1 diffusion in the plasma membrane, but that diffusion was increased greater than fourfold in protein de-enriched blebs. Cholesterol depletion in MDCK cells greatly restricted AQP1 diffusion, consistent with the formation of a network of solid-like barriers in the membrane. These results establish the nature and determinants of AQP1 diffusion in cell plasma membranes and demonstrate long-range nonanomalous diffusion of AQP1, challenging the prevailing view of universally anomalous diffusion of integral membrane proteins, and providing evidence against the accumulation of AQP1 in lipid rafts.
Figures




References
-
- Verkman, A. S. 2005. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. 118:3225–3232. - PubMed
-
- Ren, G., A. Cheng, P. Melnyk, and A. K. Mitra. 2000. Polymorphism in the packing of aquaporin-1 tetramers in 2-D crystals. J. Struct. Biol. 130:45–53. - PubMed
-
- Sui, H., B. G. Han, J. K. Lee, P. Walian, and B. K. Jap. 2001. Structural basis of water-specific transport through the AQP1 water channel. Nature. 414:872–878. - PubMed
-
- Sabolic, I., G. Valenti, J. M. Verbavatz, A. N. Van Hoek, A. S. Verkman, D. A. Ausiello, and D. Brown. 1992. Localization of the CHIP28 water channel in rat kidney. Am. J. Physiol. 263:C1225–C1233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
- GM808512/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

