Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling
- PMID: 17890448
- PMCID: PMC2200855
- DOI: 10.1182/blood-2007-06-098392
Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling
Abstract
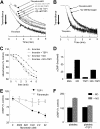
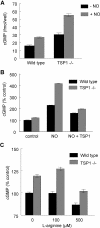
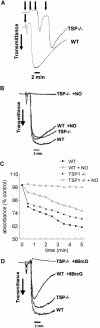
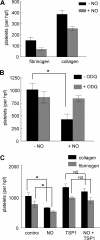
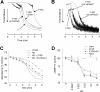
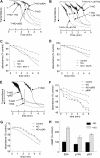
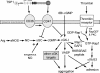
Platelet alpha-granules constitute the major rapidly releasable reservoir of thrombospondin-1 in higher animals. Although some fragments and peptides derived from thrombospondin-1 stimulate or inhibit platelet aggregation, its physiologic function in platelets has remained elusive. We now show that endogenous thrombospondin-1 is necessary for platelet aggregation in vitro in the presence of physiologic levels of nitric oxide (NO). Exogenous NO or elevation of cGMP delays thrombin-induced platelet aggregation under high shear and static conditions, and exogenous thrombospondin-1 reverses this delay. Thrombospondin-1-null murine platelets fail to aggregate in response to thrombin in the presence of exogenous NO or 8Br-cGMP. At physiologic concentrations of the NO synthase substrate arginine, thrombospondin-1-null platelets have elevated basal cGMP. Ligation of CD36 or CD47 is sufficient to block NO-induced cGMP accumulation and mimic the effect of thrombospondin-1 on aggregation. Exogenous thrombospondin-1 also reverses the suppression by NO of alphaIIb/beta3 integrin-mediated platelet adhesion on immobilized fibrinogen, mediated in part by increased GTP loading of Rap1. Thrombospondin-1 also inhibits cGMP-mediated activation of cGMP-dependent protein kinase and thereby prevents phosphorylation of VASP. Thus, release of thrombospondin-1 from alpha-granules during activation provides positive feedback to promote efficient platelet aggregation and adhesion by overcoming the antithrombotic activity of physiologic NO.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

