Blockade of the chemokine receptor CXCR2 ameliorates adjuvant-induced arthritis in rats
- PMID: 17891165
- PMCID: PMC2267272
- DOI: 10.1038/sj.bjp.0707462
Blockade of the chemokine receptor CXCR2 ameliorates adjuvant-induced arthritis in rats
Abstract
Background and purpose: Chemokine receptors CXCR1 and CXCR2 may mediate influx of neutrophils in models of acute and chronic inflammation. The potential benefits of oral administration of a CXCR1/2 inhibitor, DF 2162, in adjuvant-induced polyarthritis (AIA) were investigated.
Experimental approach: A model of AIA in rats was used to compare the therapeutic effects of the treatment with DF2162, anti-TNF or anti-CINC-1 antibodies on joint inflammation and local production of cytokines and chemokines.
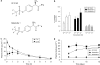
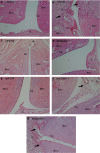
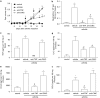
Key results: DF2162 prevented chemotaxis of rat and human neutrophils induced by chemokines acting on CXCR1/2. DF2162 was orally bioavailable and metabolized to two major metabolites. Only metabolite 1 retained CXCR1/2 blocking activity. Treatment with DF2162 (15 mg kg(-1), twice daily) or metabolite 1, but not metabolite 2, starting on day 10 after arthritis induction diminished histological score, the increase in paw volume, neutrophil influx and local production of TNF, IL-1beta, CCL2 and CCL5. The effects of DF2162 were similar to those of anti-TNF, and more effective than those of anti-CINC-1, antibodies. DF2162 prevented disease progression even when started 13 days after arthritis induction.
Conclusions and implications: DF 2162, a novel orally-active non-competitive allosteric inhibitor of CXCR1 and CXCR2, significantly ameliorates AIA in rats, an effect quantitatively and qualitatively similar to those of anti-TNF antibody treatment. These findings highlight the contribution of CXCR2 in the pathophysiology of AIA and suggest that blockade of CXCR1/2 may be a valid therapeutic target for further studies aiming at the development of new drugs for treatment of rheumatoid arthritis.
Figures
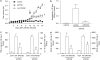


References
-
- Barcelos LS, Talvani A, Teixeira AS, Vieira LQ, Cassali GD, Andrade SP, et al. Impaired inflammatory angiogenesis, but not leukocyte influx, in mice lacking TNFR1. J Leukoc Biol. 2005;78:352–358. - PubMed
-
- Barsante MM, Roffe E, Yokoro CM, Tafuri WL, Souza DG, Pinho V, et al. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. Eur J Pharmacol. 2005;516:282–289. - PubMed
-
- Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112:139–149. - PubMed
-
- Brennan FM, Zachariae CO, Chantry D, Larsen CG, Turner M, Maini RN, et al. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990;20:2141–2144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

