Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B
- PMID: 17892322
- PMCID: PMC2323292
- DOI: 10.1371/journal.ppat.0030127
Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B
Abstract
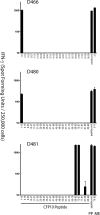
CD8(+) T cells are essential for host defense to intracellular bacterial pathogens such as Mycobacterium tuberculosis (Mtb), Salmonella species, and Listeria monocytogenes, yet the repertoire and dominance pattern of human CD8 antigens for these pathogens remains poorly characterized. Tuberculosis (TB), the disease caused by Mtb infection, remains one of the leading causes of infectious morbidity and mortality worldwide and is the most frequent opportunistic infection in individuals with HIV/AIDS. Therefore, we undertook this study to define immunodominant CD8 Mtb antigens. First, using IFN-gamma ELISPOT and synthetic peptide arrays as a source of antigen, we measured ex vivo frequencies of CD8(+) T cells recognizing known immunodominant CD4(+) T cell antigens in persons with latent tuberculosis infection. In addition, limiting dilution was used to generate panels of Mtb-specific T cell clones. Using the peptide arrays, we identified the antigenic specificity of the majority of T cell clones, defining several new epitopes. In all cases, peptide representing the minimal epitope bound to the major histocompatibility complex (MHC)-restricting allele with high affinity, and in all but one case the restricting allele was an HLA-B allele. Furthermore, individuals from whom the T cell clone was isolated harbored high ex vivo frequency CD8(+) T cell responses specific for the epitope, and in individuals tested, the epitope represented the single immunodominant response within the CD8 antigen. We conclude that Mtb-specific CD8(+) T cells are found in high frequency in infected individuals and are restricted predominantly by HLA-B alleles, and that synthetic peptide arrays can be used to define epitope specificities without prior bias as to MHC binding affinity. These findings provide an improved understanding of immunodominance in humans and may contribute to a development of an effective TB vaccine and improved immunodiagnostics.
Conflict of interest statement
Figures




References
-
- WHO report 2007. Geneva: World Health Organization; 2007. Global tuberculosis control: Surveillance, planning, financing.
-
- The global plan to stop TB, 2006–2015 / Stop TB Partnership. Geneva: World Health Organization; 2006.
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. - PubMed
-
- Oldstone MB. The role of cytotoxic T lymphocytes in infectious disease: History, criteria, and state of the art. Curr Top Microbiol Immunol. 1994;189:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

