Hypoxia attenuates effector-target cell interaction in the airway and pulmonary vascular compartment
- PMID: 17892511
- PMCID: PMC2219348
- DOI: 10.1111/j.1365-2249.2007.03495.x
Hypoxia attenuates effector-target cell interaction in the airway and pulmonary vascular compartment
Abstract
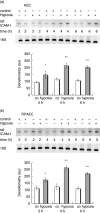
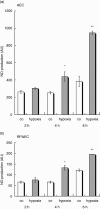
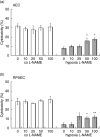
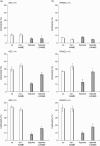
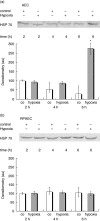
Leucocyte infiltration is known to play an important role in hypoxia-induced tissue damage. However, little information is available about hypoxia and interaction of effector (neutrophils) with target cells (alveolar epithelial cells, AEC; rat pulmonary artery endothelial cells, RPAEC). The goal of this study was to elucidate hypoxia-induced changes of effector-target cell interaction. AEC and RPAEC were exposed to 5% oxygen for 2-6 h. Intercellular adhesion molecule-1 (ICAM-1) expression was determined and cell adherence as well as cytotoxicity assays were performed. Nitric oxide and heat shock protein 70 (HSP70) production was assessed in target cells. Under hypoxic conditions enhanced ICAM-1 production was found in both cell types. This resulted in an increase of adherent neutrophils to AEC and RPAEC. The death rate of hypoxia-exposed target cells decreased significantly in comparison to control cells. Nitric oxide (NO) concentration was enhanced, as was production of HSP70 in AEC. Blocking NO production in target cells resulted in increased cytotoxicity in AEC and RPAEC. This study shows for the first time that target cells are more resistant to effector cells under hypoxia, suggesting hypoxia-induced cell protection. An underlying mechanism for this phenomenon might be the protective effect of increased levels of NO in target cells.
Figures


References
-
- Beck-Schimmer B, Schimmer RC, Madjdpour C, Bonvini JM, Pasch T, Ward PA. Hypoxia mediates increased neutrophil and macrophage adhesiveness to alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:780–7. - PubMed
-
- Kuo HP, Lin HC, Hwang KH, Wang CH, Lu LC. Lipopolysaccharide enhances substance P-mediated neutrophil adherence to epithelial cells and cytokine release. Am J Respir Crit Care Med. 2000;162:1891–7. - PubMed
-
- Beck-Schimmer B, Schimmer RC, Warner RL, et al. Expression of lung vascular and airway ICAM-1 after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 1997;17:344–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

