Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis
- PMID: 17892536
- PMCID: PMC2375038
- DOI: 10.1186/gb-2007-8-9-r200
Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis
Abstract
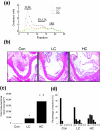
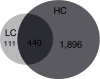
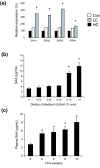
Background: Increased dietary cholesterol intake is associated with atherosclerosis. Atherosclerosis development requires a lipid and an inflammatory component. It is unclear where and how the inflammatory component develops. To assess the role of the liver in the evolution of inflammation, we treated ApoE*3Leiden mice with cholesterol-free (Con), low (LC; 0.25%) and high (HC; 1%) cholesterol diets, scored early atherosclerosis and profiled the (patho)physiological state of the liver using novel whole-genome and metabolome technologies.
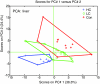
Results: Whereas the Con diet did not induce early atherosclerosis, the LC diet did so but only mildly, and the HC diet induced it very strongly. With increasing dietary cholesterol intake, the liver switches from a resilient, adaptive state to an inflammatory, pro-atherosclerotic state. The liver absorbs moderate cholesterol stress (LC) mainly by adjusting metabolic and transport processes. This hepatic resilience is predominantly controlled by SREBP-1/-2, SP-1, RXR and PPARalpha. A further increase of dietary cholesterol stress (HC) additionally induces pro-inflammatory gene expression, including pro-atherosclerotic candidate genes. These HC-evoked changes occur via specific pro-inflammatory pathways involving specific transcriptional master regulators, some of which are established, others newly identified. Notably, several of these regulators control both lipid metabolism and inflammation, and thereby link the two processes.
Conclusion: With increasing dietary cholesterol intake the liver switches from a mainly resilient (LC) to a predominantly inflammatory (HC) state, which is associated with early lesion formation. Newly developed, functional systems biology tools allowed the identification of novel regulatory pathways and transcriptional regulators controlling both lipid metabolism and inflammatory responses, thereby providing a rationale for an interrelationship between the two processes.
Figures


References
-
- Blum CB, Levy RI. Role of dietary intervention in the primary prevention of coronary heart disease. Individuals with high-normal or elevated serum cholesterol levels should be placed on cholesterol-lowering diets. Cardiology. 1987;74:2–21. - PubMed
-
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

