Base excision repair of ionizing radiation-induced DNA damage in G1 and G2 cell cycle phases
- PMID: 17892593
- PMCID: PMC2063494
- DOI: 10.1186/1475-2867-7-15
Base excision repair of ionizing radiation-induced DNA damage in G1 and G2 cell cycle phases
Abstract
Background: Major genomic surveillance mechanisms regulated in response to DNA damage exist at the G1/S and G2/M checkpoints. It is presumed that these delays provide time for the repair of damaged DNA. Cells have developed multiple DNA repair pathways to protect themselves from different types of DNA damage. Oxidative DNA damage is processed by the base excision repair (BER) pathway. Little is known about the BER of ionizing radiation-induced DNA damage and putative heterogeneity of BER in the cell cycle context. We measured the activities of three BER enzymes throughout the cell cycle to investigate the cell cycle-specific repair of ionizing radiation-induced DNA damage. We further examined BER activities in G2 arrested human cells after exposure to ionizing radiation.
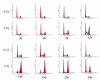
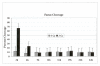
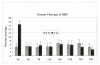
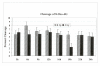
Results: Using an in vitro incision assay involving radiolabeled oligonucleotides with specific DNA lesions, we examined the activities of several BER enzymes in the whole cell extracts prepared from synchronized human HeLa cells irradiated in G1 and G2 phase of the cell cycle. The activities of human endonuclease III (hNTH1), a glycosylase/lyase that removes several damaged bases from DNA including dihydrouracil (DHU), 8-oxoguanine-DNA glycosylase (hOGG1) that recognizes 7,8-dihydro-8-oxo-2'-deoxyguanosine (8-oxoG) lesion and apurinic/apyrimidinic endonuclease (hAPE1) that acts on abasic sites including synthetic analog furan were examined.
Conclusion: Overall the repair activities of hNTH1 and hAPE1 were higher in the G1 compared to G2 phase of the cell cycle. The percent cleavages of oligonucleotide substrate with furan were greater than substrate with DHU in both G1 and G2 phases. The irradiation of cells enhanced the cleavage of substrates with furan and DHU only in G1 phase. The activity of hOGG1 was much lower and did not vary within the cell cycle. These results demonstrate the cell cycle phase dependence on the BER of ionizing radiation-induced DNA damage. Interestingly no evidence of enhanced BER activities was found in irradiated cells arrested in G2 phase.
Figures
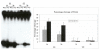
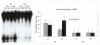
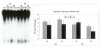

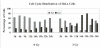
References
-
- Reardon JT, Bessho T, Kung HC, Bolton PH, Sancar A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc Natl Acad Sci U S A. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. - DOI - PMC - PubMed
-
- D'Errico M, Parlanti E, Teson M, de Jesus BM, Degan P, Calcagnile A, Jaruga P, Bjoras M, Crescenzi M, Pedrini AM, Egly JM, Zambruno G, Stefanini M, Dizdaroglu M, Dogliotti E. New functions of XPC in the protection of human skin cells from oxidative damage. Embo J. 2006;25:4305–4315. doi: 10.1038/sj.emboj.7601277. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

