NF-kappaB inhibitor dehydroxymethylepoxyquinomicin suppresses osteoclastogenesis and expression of NFATc1 in mouse arthritis without affecting expression of RANKL, osteoprotegerin or macrophage colony-stimulating factor
- PMID: 17892600
- PMCID: PMC2212584
- DOI: 10.1186/ar2298
NF-kappaB inhibitor dehydroxymethylepoxyquinomicin suppresses osteoclastogenesis and expression of NFATc1 in mouse arthritis without affecting expression of RANKL, osteoprotegerin or macrophage colony-stimulating factor
Abstract
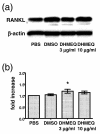
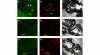
Inhibition of NF-kappaB is known to be effective in reducing both inflammation and bone destruction in animal models of arthritis. Our previous study demonstrated that a small cell-permeable NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin (DHMEQ), suppresses expression of proinflammatory cytokines and ameliorates mouse arthritis. It remained unclear, however, whether DHMEQ directly affects osteoclast precursor cells to suppress their differentiation to mature osteoclasts in vivo. The effect of DHMEQ on human osteoclastogenesis also remained elusive. In the present study, we therefore examined the effect of DHMEQ on osteoclastogenesis using a mouse collagen-induced arthritis model, and using culture systems of fibroblast-like synovial cells obtained from patients with rheumatoid arthritis, and of osteoclast precursor cells from peripheral blood of healthy volunteers. DHMEQ significantly suppressed formation of osteoclasts in arthritic joints, and also suppressed expression of NFATc1 along the inner surfaces of bone lacunae and the eroded bone surface, while serum levels of soluble receptor activator of NF-kappaB ligand (RANKL), osteoprotegerin and macrophage colony-stimulating factor were not affected by the treatment. DHMEQ also did not suppress spontaneous expression of RANKL nor of macrophage colony-stimulating factor in culture of fibroblast-like synovial cells obtained from patients with rheumatoid arthritis. These results suggest that DHMEQ suppresses osteoclastogenesis in vivo, through downregulation of NFATc1 expression, without significantly affecting expression of upstream molecules of the RANKL/receptor activator of NF-kappaB/osteoprotegerin cascade, at least in our experimental condition. Furthermore, in the presence of RANKL and macrophage colony-stimulating factor, differentiation and activation of human osteoclasts were also suppressed by DHMEQ, suggesting the possibility of future application of NF-kappaB inhibitors to rheumatoid arthritis therapy.
Figures





References
-
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. - DOI - PubMed
-
- van der Heijde D, Klareskog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, Tornero-Molina J, Wajdula J, Pedersen R, Fatenejad S. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis. Two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–1074. doi: 10.1002/art.21655. - DOI - PubMed
-
- Smolen JS, van der Heijde DMFM, St Clair EW, Emery P, Bathon JM, Keystone E, Maini RN, Kalden JR, Schiff M, Baker D, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab. Results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–710. doi: 10.1002/art.21678. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

