Molecular determinant for specific Ca/Ba selectivity profiles of low and high threshold Ca2+ channels
- PMID: 17893194
- PMCID: PMC2151654
- DOI: 10.1085/jgp.200709771
Molecular determinant for specific Ca/Ba selectivity profiles of low and high threshold Ca2+ channels
Abstract
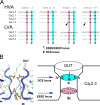
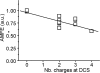
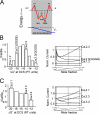
Voltage-gated Ca(2+) channels (VGCC) play a key role in many physiological functions by their high selectivity for Ca(2+) over other divalent and monovalent cations in physiological situations. Divalent/monovalent selection is shared by all VGCC and is satisfactorily explained by the existence, within the pore, of a set of four conserved glutamate/aspartate residues (EEEE locus) coordinating Ca(2+) ions. This locus however does not explain either the choice of Ca(2+) among other divalent cations or the specific conductances encountered in the different VGCC. Our systematic analysis of high- and low-threshold VGCC currents in the presence of Ca(2+) and Ba(2+) reveals highly specific selectivity profiles. Sequence analysis, molecular modeling, and mutational studies identify a set of nonconserved charged residues responsible for these profiles. In HVA (high voltage activated) channels, mutations of this set modify divalent cation selectivity and channel conductance without change in divalent/monovalent selection, activation, inactivation, and kinetics properties. The Ca(V)2.1 selectivity profile is transferred to Ca(V)2.3 when exchanging their residues at this location. Numerical simulations suggest modification in an external Ca(2+) binding site in the channel pore directly involved in the choice of Ca(2+), among other divalent physiological cations, as the main permeant cation for VGCC. In LVA (low voltage activated) channels, this locus (called DCS for divalent cation selectivity) also influences divalent cation selection, but our results suggest the existence of additional determinants to fully recapitulate all the differences encountered among LVA channels. These data therefore attribute to the DCS a unique role in the specific shaping of the Ca(2+) influx between the different HVA channels.
Figures




Similar articles
-
Localization of divalent cation-binding site in the pore of a small conductance Ca(2+)-activated K(+) channel and its role in determining current-voltage relationship.Biophys J. 2002 Nov;83(5):2528-38. doi: 10.1016/S0006-3495(02)75264-8. Biophys J. 2002. PMID: 12414687 Free PMC article.
-
Glutamate substitution in repeat IV alters divalent and monovalent cation permeation in the heart Ca2+ channel.Biophys J. 1995 Nov;69(5):1801-13. doi: 10.1016/S0006-3495(95)80050-0. Biophys J. 1995. PMID: 8580323 Free PMC article.
-
A novel molecular inactivation determinant of voltage-gated CaV1.2 L-type Ca2+ channel.Neuroscience. 2006;139(4):1275-87. doi: 10.1016/j.neuroscience.2006.01.028. Epub 2006 Mar 14. Neuroscience. 2006. PMID: 16533566
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
Molecular pore structure of voltage-gated sodium and calcium channels.Braz J Med Biol Res. 1994 Dec;27(12):2781-802. Braz J Med Biol Res. 1994. PMID: 7550000 Review.
Cited by
-
Permeation and gating in CaV3.1 (alpha1G) T-type calcium channels effects of Ca2+, Ba2+, Mg2+, and Na+.J Gen Physiol. 2008 Aug;132(2):223-38. doi: 10.1085/jgp.200809986. J Gen Physiol. 2008. PMID: 18663131 Free PMC article.
-
Honeybee CaV4 has distinct permeation, inactivation, and pharmacology from homologous NaV channels.J Gen Physiol. 2024 May 6;156(5):e202313509. doi: 10.1085/jgp.202313509. Epub 2024 Apr 1. J Gen Physiol. 2024. PMID: 38557788 Free PMC article.
-
Honeybee locomotion is impaired by Am-CaV3 low voltage-activated Ca2+ channel antagonist.Sci Rep. 2017 Feb 1;7:41782. doi: 10.1038/srep41782. Sci Rep. 2017. PMID: 28145504 Free PMC article.
-
Deciphering Ca2+ permeation and valence selectivity in CaV1: Molecular dynamics simulations reveal the three-ion knock-on mechanism.Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2424694122. doi: 10.1073/pnas.2424694122. Epub 2025 May 29. Proc Natl Acad Sci U S A. 2025. PMID: 40440072 Free PMC article.
-
A single amino acid change in Ca(v)1.2 channels eliminates the permeation and gating differences between Ca(2+) and Ba(2+).J Membr Biol. 2010 Feb;233(1-3):23-33. doi: 10.1007/s00232-009-9221-1. Epub 2010 Jan 23. J Membr Biol. 2010. PMID: 20098982 Free PMC article.
References
-
- Catterall, W.A. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16:521–555. - PubMed

