Staphylococcal complement evasion by various convertase-blocking molecules
- PMID: 17893203
- PMCID: PMC2118443
- DOI: 10.1084/jem.20070818
Staphylococcal complement evasion by various convertase-blocking molecules
Abstract
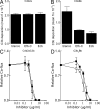
To combat the human immune response, bacteria should be able to divert the effectiveness of the complement system. We identify four potent complement inhibitors in Staphylococcus aureus that are part of a new immune evasion cluster. Two are homologues of the C3 convertase modulator staphylococcal complement inhibitor (SCIN) and function in a similar way as SCIN. Extracellular fibrinogen-binding protein (Efb) and its homologue extracellular complement-binding protein (Ecb) are identified as potent complement evasion molecules, and their inhibitory mechanism was pinpointed to blocking C3b-containing convertases: the alternative pathway C3 convertase C3bBb and the C5 convertases C4b2aC3b and C3b2Bb. The potency of Efb and Ecb to block C5 convertase activity was demonstrated by their ability to block C5a generation and C5a-mediated neutrophil activation in vitro. Further, Ecb blocks C5a-dependent neutrophil recruitment into the peritoneal cavity in a mouse model of immune complex peritonitis. The strong antiinflammatory properties of these novel S. aureus-derived convertase inhibitors make these compounds interesting drug candidates for complement-mediated diseases.
Figures








References
-
- Neth, O., D.L. Jack, M. Johnson, N.J. Klein, and M.W. Turner. 2002. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J. Immunol. 169:4430–4436. - PubMed
-
- Wergeland, H., C. Endresen, O.B. Natas, P. Aasjord, and P. Oeding. 1984. Antibodies to Staphylococcus aureus peptidoglycan and lipoteichoic acid in sera from blood donors and patients with staphylococcal infections. Acta Pathol. Microbiol. Immunol. Scand. [B]. 92:265–269. - PubMed
-
- Walport, M.J. 2001. Complement. Second of two parts. N. Engl. J. Med. 344:1140–1144. - PubMed
-
- Walport, M.J. 2001. Complement. First of two parts. N. Engl. J. Med. 344:1058–1066. - PubMed
-
- Muller-Eberhard, H.J. 1986. The membrane attack complex of complement. Annu. Rev. Immunol. 4:503–528. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

