Aquatic eutrophication promotes pathogenic infection in amphibians
- PMID: 17893332
- PMCID: PMC2000446
- DOI: 10.1073/pnas.0707763104
Aquatic eutrophication promotes pathogenic infection in amphibians
Abstract
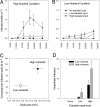
The widespread emergence of human and wildlife diseases has challenged ecologists to understand how large-scale agents of environmental change affect host-pathogen interactions. Accelerated eutrophication of aquatic ecosystems owing to nitrogen and phosphorus enrichment is a pervasive form of environmental change that has been implicated in the emergence of diseases through direct and indirect pathways. We provide experimental evidence linking eutrophication and disease in a multihost parasite system. The trematode parasite Ribeiroia ondatrae sequentially infects birds, snails, and amphibian larvae, frequently causing severe limb deformities and mortality. Eutrophication has been implicated in the emergence of this parasite, but definitive evidence, as well as a mechanistic understanding, have been lacking until now. We show that the effects of eutrophication cascade through the parasite life cycle to promote algal production, the density of snail hosts, and, ultimately, the intensity of infection in amphibians. Infection also negatively affected the survival of developing amphibians. Mechanistically, eutrophication promoted amphibian disease through two distinctive pathways: by increasing the density of infected snail hosts and by enhancing per-snail production of infectious parasites. Given forecasted increases in global eutrophication, amphibian extinctions, and similarities between Ribeiroia and important human and wildlife pathogens, our results have broad epidemiological and ecological significance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Science. 2002;296:2160–2162. - PubMed
-
- Pope K, Masuoka P, Rejmánková E, Grieco J, Johnson S, Roberts D. Ecol Appl. 2005;15:1223–1232.
-
- Patz JS, Campbell-Lendrum D, Holloway T, Foley JA. Nature. 2005;438:310–317. - PubMed
-
- Daszak P, Cunningham AA, Hyatt AD. Science. 2000;287:443–449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

