Physical plasticity of the nucleus in stem cell differentiation
- PMID: 17893336
- PMCID: PMC2000408
- DOI: 10.1073/pnas.0702576104
Physical plasticity of the nucleus in stem cell differentiation
Abstract
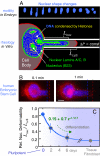
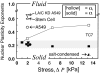
Cell differentiation in embryogenesis involves extensive changes in gene expression structural reorganization within the nucleus, including chromatin condensation and nucleoprotein immobilization. We hypothesized that nuclei in naive stem cells would therefore prove to be physically plastic and also more pliable than nuclei in differentiated cells. Micromanipulation methods indeed show that nuclei in human embryonic stem cells are highly deformable and stiffen 6-fold through terminal differentiation, and that nuclei in human adult stem cells possess an intermediate stiffness and deform irreversibly. Because the nucleo-skeletal component Lamin A/C is not expressed in either type of stem cell, we knocked down Lamin A/C in human epithelial cells and measured a deformability similar to that of adult hematopoietic stem cells. Rheologically, lamin-deficient states prove to be the most fluid-like, especially within the first approximately 10 sec of deformation. Nuclear distortions that persist longer than this are irreversible, and fluorescence-imaged microdeformation with photobleaching confirms that chromatin indeed flows, distends, and reorganizes while the lamina stretches. The rheological character of the nucleus is thus set largely by nucleoplasm/chromatin, whereas the extent of deformation is modulated by the lamina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

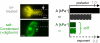



References
-
- Blau H, Pavlath G, Hardeman E, Chiu C, Silberstein L, Webster S, Miller S, Webster C. Science. 1985;230:758–766. - PubMed
-
- Slack JMW, Tosh D. Curr Opin Genet Dev. 2001;11:581–586. - PubMed
-
- Szutorisz H, Dillon N. BioEssays. 2005;27:1286–1293. - PubMed
-
- Labrador M, Corces VG. Cell. 2002;111:151–154. - PubMed
-
- West AG, Fraser P. Hum Mol Genet. 2005;14:R101–R111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

