Solution structure and proposed domain domain recognition interface of an acyl carrier protein domain from a modular polyketide synthase
- PMID: 17893358
- PMCID: PMC2204127
- DOI: 10.1110/ps.073011407
Solution structure and proposed domain domain recognition interface of an acyl carrier protein domain from a modular polyketide synthase
Abstract
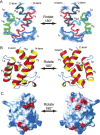
Polyketides are a medicinally important class of natural products. The architecture of modular polyketide synthases (PKSs), composed of multiple covalently linked domains grouped into modules, provides an attractive framework for engineering novel polyketide-producing assemblies. However, impaired domain-domain interactions can compromise the efficiency of engineered polyketide biosynthesis. To facilitate the study of these domain-domain interactions, we have used nuclear magnetic resonance (NMR) spectroscopy to determine the first solution structure of an acyl carrier protein (ACP) domain from a modular PKS, 6-deoxyerythronolide B synthase (DEBS). The tertiary fold of this 10-kD domain is a three-helical bundle; an additional short helix in the second loop also contributes to the core helical packing. Superposition of residues 14-94 of the ensemble on the mean structure yields an average atomic RMSD of 0.64 +/- 0.09 Angstrom for the backbone atoms (1.21 +/- 0.13 Angstrom for all non-hydrogen atoms). The three major helices superimpose with a backbone RMSD of 0.48 +/- 0.10 Angstrom (0.99 +/- 0.11 Angstrom for non-hydrogen atoms). Based on this solution structure, homology models were constructed for five other DEBS ACP domains. Comparison of their steric and electrostatic surfaces at the putative interaction interface (centered on helix II) suggests a model for protein-protein recognition of ACP domains, consistent with the previously observed specificity. Site-directed mutagenesis experiments indicate that two of the identified residues influence the specificity of ACP recognition.
Figures




References
-
- Broadhurst R.W., Nietlispach, D., Wheatcroft, M.P., Leadlay, P.F., and Weissman, K.J. 2003. The structure of docking domains in modular polyketide synthases. Chem. Biol. 10: 723–731. - PubMed
-
- Cane D.E., Walsh, C.T., and Khosla, C. 1998. Harnessing the biosynthetic code: Combinations, permutations, and mutations. Science 282: 63–68. - PubMed
-
- Cortes J., Haydock, S.F., Roberts, G.A., Bevitt, D.J., and Leadlay, P.F. 1990. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea . Nature 348: 176–178. - PubMed
-
- Crump M.P., Crosby, J., Dempsey, C.E., Parkinson, J.A., Murray, M., Hopwood, D.A., and Simpson, T.J. 1997. Solution structure of the actinorhodin polyketide synthase acyl carrier protein from Streptomyces coelicolor A3(2) . Biochemistry 36: 6000–6008. - PubMed
-
- Donadio S. and Katz, L. 1992. Organization of the enzymatic domains in the multifunctional polyketide synthase involved in erythromycin formation in Saccharopolyspora erythraea . Gene 111: 51–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

