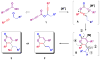
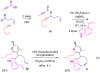
Complex alpha-pyrones synthesized by a gold-catalyzed coupling reaction
- PMID: 17893896
- PMCID: PMC2790064
- DOI: 10.1002/anie.200703276
Complex alpha-pyrones synthesized by a gold-catalyzed coupling reaction
Figures


Similar articles
-
Syntheses of α-pyrones using gold-catalyzed coupling reactions.Org Lett. 2011 Jun 3;13(11):2834-6. doi: 10.1021/ol200794w. Epub 2011 May 2. Org Lett. 2011. PMID: 21534543 Free PMC article.
-
Gold-catalyzed sequential alkyne activation for the synthesis of 4,6-disubstituted phosphorus 2-pyrones.Org Lett. 2013 Jan 4;15(1):26-9. doi: 10.1021/ol3029274. Epub 2012 Dec 13. Org Lett. 2013. PMID: 23236965
-
Total synthesis of neurymenolide A based on a gold-catalyzed synthesis of 4-hydroxy-2-pyrones.Angew Chem Int Ed Engl. 2012 Jul 9;51(28):6929-33. doi: 10.1002/anie.201203180. Epub 2012 Jun 4. Angew Chem Int Ed Engl. 2012. PMID: 22674881
-
Gold-catalyzed selective oxidation of 4-oxahepta-1,6-diynes to 2H-pyran-3(6H)-ones and chromen-3(4H)-ones via β-gold vinyl cation intermediates.Chem Commun (Camb). 2015 Jun 28;51(51):10318-21. doi: 10.1039/c5cc02952j. Chem Commun (Camb). 2015. PMID: 26023766
-
Recent advances in the synthesis of 2-pyrones.Mar Drugs. 2015 Mar 23;13(3):1581-620. doi: 10.3390/md13031581. Mar Drugs. 2015. PMID: 25806468 Free PMC article. Review.
Cited by
-
Base-Promoted Selective Synthesis of 2H-Pyranones and Tetrahydronaphthalenes via Domino Reactions.ACS Omega. 2017 Aug 24;2(8):4900-4910. doi: 10.1021/acsomega.7b00627. eCollection 2017 Aug 31. ACS Omega. 2017. PMID: 31457769 Free PMC article.
-
Mechanistic studies on Au(I)-catalyzed [3,3]-sigmatropic rearrangements using cyclopropane probes.J Am Chem Soc. 2009 Apr 1;131(12):4513-20. doi: 10.1021/ja900456m. J Am Chem Soc. 2009. PMID: 19275228 Free PMC article.
-
Gold(I)-catalyzed coupling reactions for the synthesis of diverse small molecules using the build/couple/pair strategy.J Am Chem Soc. 2009 Apr 22;131(15):5667-74. doi: 10.1021/ja900414s. J Am Chem Soc. 2009. PMID: 19331418 Free PMC article.
-
Alkynyl triazenes enable divergent syntheses of 2-pyrones.Chem Sci. 2021 Jun 4;12(26):9140-9145. doi: 10.1039/d1sc02583j. eCollection 2021 Jul 7. Chem Sci. 2021. PMID: 34276943 Free PMC article.
-
Syntheses of α-pyrones using gold-catalyzed coupling reactions.Org Lett. 2011 Jun 3;13(11):2834-6. doi: 10.1021/ol200794w. Epub 2011 May 2. Org Lett. 2011. PMID: 21534543 Free PMC article.
References
-
-
For examples, see: Kumagai N, Muncipinto G, Schreiber SL. Angew Chem. 2006;118:3717–3720.Angew Chem Int Ed. 2006;45:3635–3638.Spiegel DA, Schroeder FC, Duvall JR, Schreiber SL. J Am Chem Soc. 2006;128:14766–14767.
-
-
- Nielsen TE, Schreiber SL. submitted.
-
- Dickinson JM. Nat Prod Rep. 1993;10:71–98. - PubMed
- Steyn PS, van Heerden FR. Nat Prod Rep. 1998;15:397–413. - PubMed
- McGlacken GP, Fairlamb IJ. Nat Prod Rep. 2005;22:369–385. - PubMed
- Salomon CE, Magarvey NA, Sherman DH. Nat Prod Rep. 2004;21:105–121. - PubMed
- van Raaij MJ, Abrahams JP, Leslie AG, Walker JE. Proc Natl Acad Sci U S A. 1996;93:6913–6917. - PMC - PubMed
- Wu PL, Hsu YL, Wu TS, Bastow KF, Lee KH. Org Lett. 2006;8:5207–5210. - PubMed
- Clark BR, Capon RJ, Lacey E, Tennant S, Gill JH. Org Lett. 2006;8:701–704. - PubMed
- Lee JC, Lobkovsky E, Pliam NB, Strobel G, Clardy J. J Org Chem. 1995;60:7076–7077.
- Thaisrivongs S, Janakiraman MN, Chong KT, Tomich PK, Dolak LA, Turner SR, Strohbach JW, Lynn JC, Horng MM, Hinshaw RR, Watenpaugh KD. J Med Chem. 1996;39:2400–2410. - PubMed
-
- Jung ME, Hagenah JA. J Org Chem. 1987;52:1889–1902.
- Dieter RK, Fishpaugh JR. J Org Chem. 1988;53:2031–2046.
- Douglas CJ, Sklenicka HM, Shen HC, Mathias DS, Degen SJ, Golding GM, Morgan CD, Shih RA, Mueller KL, Scurer LM, Johnson EW, Hsung RP. Tetrahedron. 1999;55:13683–13696.
- Ma S, Yu S, Yin S. J Org Chem. 2003;68:8996–9002. - PubMed
- Hachiya I, Shibuya H, Shimizu M. Tetrahedron Lett. 2003;44:2061–2063.
- Gilbreath SG, Harris CM, Harris TM. J Am Chem Soc. 1988;110:6172–6179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

