Translocation of particles and inflammatory responses after exposure to fine particles and nanoparticles in an epithelial airway model
- PMID: 17894871
- PMCID: PMC2039730
- DOI: 10.1186/1743-8977-4-9
Translocation of particles and inflammatory responses after exposure to fine particles and nanoparticles in an epithelial airway model
Abstract
Background: Experimental studies provide evidence that inhaled nanoparticles may translocate over the airspace epithelium and cause increased cellular inflammation. Little is known, however, about the dependence of particle size or material on translocation characteristics, inflammatory response and intracellular localization.
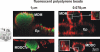
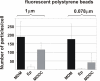
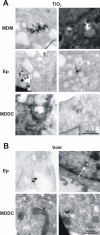
Results: Using a triple cell co-culture model of the human airway wall composed of epithelial cells, macrophages and dendritic cells we quantified the entering of fine (1 mum) and nano-sized (0.078 mum) polystyrene particles by laser scanning microscopy. The number distribution of particles within the cell types was significantly different between fine and nano-sized particles suggesting different translocation characteristics. Analysis of the intracellular localization of gold (0.025 mum) and titanium dioxide (0.02-0.03 mum) nanoparticles by energy filtering transmission electron microscopy showed differences in intracellular localization depending on particle composition. Titanium dioxide nanoparticles were detected as single particles without membranes as well as in membrane-bound agglomerations. Gold nanoparticles were found inside the cells as free particles only. The potential of the different particle types (different sizes and different materials) to induce a cellular response was determined by measurements of the tumour necrosis factor-alpha in the supernatants. We measured a 2-3 fold increase of tumour necrosis factor-alpha in the supernatants after applying 1 mum polystyrene particles, gold nanoparticles, but not with polystyrene and titanium dioxide nanoparticles.
Conclusion: Quantitative laser scanning microscopy provided evidence that the translocation and entering characteristics of particles are size-dependent. Energy filtering transmission electron microscopy showed that the intracellular localization of nanoparticles depends on the particle material. Both particle size and material affect the cellular responses to particle exposure as measured by the generation of tumour necrosis factor-alpha.
Figures

References
-
- Maynard A, Michelson E. The Nanotechnology Consumer Products Inventory. 2005. http://www.nanotechproject.org/index.php?id=44

