Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling
- PMID: 17895382
- PMCID: PMC2000402
- DOI: 10.1073/pnas.0705749104
Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling
Abstract
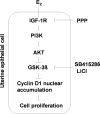
Estradiol-17beta (E(2)) causes cell proliferation in the uterine epithelium of mice and humans by signaling through its transcription factor receptor alpha (ERalpha). In this work we show that this signaling is mediated by the insulin-like growth factor 1 receptor (IGF1R) expressed in the epithelium, whose activation leads to the stimulation of the phosphoinositide 3-kinase/protein kinase B pathway leading to cyclin D1 nuclear accumulation and engagement with the canonical cell cycle machinery. This cyclin D1 nuclear accumulation results from the inhibition of glycogen synthase kinase 3beta (GSK3beta) activity caused by an inhibitory phosphorylation by protein kinase B. Once the IGF1 pathway is activated, inhibition of ER signaling demonstrates that it is independent of ER. Inhibition of GSK3beta in the absence of E(2) is sufficient to induce uterine epithelial cell proliferation, and GSK3beta is epistatic to IGF1 signaling, indicating a linear pathway from E(2) to cyclin D1. Exposure to E(2) is the major risk factor for endometrial cancer, suggesting that downstream activation of this IGF1-mediated pathway by mutation could be causal in the progression to ER-independent tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation.Mol Endocrinol. 2005 Aug;19(8):1978-90. doi: 10.1210/me.2004-0274. Epub 2005 Apr 21. Mol Endocrinol. 2005. PMID: 15845746
-
Cyclin D2 compensates for the loss of cyclin D1 in estrogen-induced mouse uterine epithelial cell proliferation.Mol Endocrinol. 2003 Jul;17(7):1368-81. doi: 10.1210/me.2003-0036. Epub 2003 Mar 20. Mol Endocrinol. 2003. PMID: 12649329
-
Autocrine stimulation of IGF1 in estrogen-induced growth of endometrial carcinoma cells: involvement of the mitogen-activated protein kinase pathway followed by up-regulation of cyclin D1 and cyclin E.Endocr Relat Cancer. 2009 Mar;16(1):113-22. doi: 10.1677/ERC-08-0117. Epub 2008 Oct 13. Endocr Relat Cancer. 2009. PMID: 18852162
-
GSK-3beta mediates in the progesterone inhibition of estrogen induced cyclin D2 nuclear localization and cell proliferation in cyclin D1-/- mouse uterine epithelium.FEBS Lett. 2007 Jun 26;581(16):3069-75. doi: 10.1016/j.febslet.2007.05.072. Epub 2007 Jun 4. FEBS Lett. 2007. PMID: 17560576
-
Mechanism of estrogen action: lessons from the estrogen receptor-alpha knockout mouse.Biol Reprod. 1998 Sep;59(3):470-5. doi: 10.1095/biolreprod59.3.470. Biol Reprod. 1998. PMID: 9716542 Review. No abstract available.
Cited by
-
Inhibition of oxygen-induced hypoxia-inducible factor-1alpha degradation unmasks estradiol induction of vascular endothelial growth factor expression in ECC-1 cancer cells in vitro.Endocrinology. 2009 Dec;150(12):5405-14. doi: 10.1210/en.2009-0884. Epub 2009 Oct 9. Endocrinology. 2009. PMID: 19819950 Free PMC article.
-
Inside the Endometrial Cell Signaling Subway: Mind the Gap(s).Int J Mol Sci. 2018 Aug 21;19(9):2477. doi: 10.3390/ijms19092477. Int J Mol Sci. 2018. PMID: 30134622 Free PMC article. Review.
-
Mechanisms of uterine estrogen signaling during early pregnancy in mice: an update.J Mol Endocrinol. 2016 Apr;56(3):R127-38. doi: 10.1530/JME-15-0300. Epub 2016 Feb 17. J Mol Endocrinol. 2016. PMID: 26887389 Free PMC article. Review.
-
Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in vivo?Front Endocrinol (Lausanne). 2020 Mar 31;11:148. doi: 10.3389/fendo.2020.00148. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32296387 Free PMC article. Review.
-
MCM2 mediates progesterone-induced endometrial stromal cell proliferation and differentiation in mice.Endocrine. 2016 Aug;53(2):595-606. doi: 10.1007/s12020-016-0894-9. Epub 2016 Feb 24. Endocrine. 2016. PMID: 26910396
References
-
- Tong W, Pollard JW. In: The Endometrium. Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. London: Taylor and Francis; 2002. pp. 94–109.
-
- Brinton LA, Lacey JV, Jr, Trimble EL. Lancet. 2005;365:1517–1518. - PubMed
-
- Sherr CJ. Cell. 1994;79:551–555. - PubMed
-
- Sherr CJ, Roberts JM. Genes Dev. 1995;9:1149–1163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

