Thermal injury-plus-sepsis contributes to a substantial deletion of intestinal mesenteric lymph node CD4 T cell via apoptosis
- PMID: 17895960
- PMCID: PMC1989035
- DOI: 10.7150/ijbs.3.393
Thermal injury-plus-sepsis contributes to a substantial deletion of intestinal mesenteric lymph node CD4 T cell via apoptosis
Abstract
Thermal injury (TI) with septic complications continues to be a serious clinical problem. One of the main concerns in such patients is immunosuppression related to functional derangements in intestinal CD4+ T lymphocytes. Extensive previous studies in thermal injury/septic patients and animal models of thermal injury/sepsis have shown decreased responsiveness of intestinal CD4+ T cells to antigen/mitogen. This hyporesponsiveness could significantly contribute to increase injured host susceptibility to pathogens including those translocating from host's gut lumen. Our previous studies indicated that while thermal injury or sepsis alone lead to suppressed proliferation and IL-2 production of intestinal CD4+ T cells, this study showed a substantial deletion via apoptosis of the Mesenteric Lymph Nodes (MLN) CD4+ T cells. Hence, thermal injury-plus-sepsis contributes not only to suppressed CD4+ T proliferation/IL-2 production but also to a substantial modulation of CD4+ T cell survivability. These findings allow us to conclude that while thermal injury alone can produce attenuated cell mediated responses without an overt change in CD4+ T cell survival, thermal injury with septic complications causes CD4+ T cell death and an irreversible loss of cell-mediated responses. The latter happening could be responsible for high morbidity and mortality in the injured host afflicted with thermal injury plus a critical infection.
Keywords: Burn injury; activation-induced cell death; adaptive immune response; hoescht staining; immune suppression; infection.
Conflict of interest statement
Conflict of interest: The authors declare that they do not have any conflict of interest in this study.
Figures




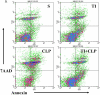


References
-
- Vandijck DM, Brusselaers N, Blot SI. Septicemia as a cause of death in burns. Burns. 2007;33(4):538–9. - PubMed
-
- Purdue GF. American Burn Association Presidential Address 2006 on Nutrition: yesterday, today, and tomorrow. J Burn Care Res. 2007;28(1):1–5. - PubMed
-
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunctions. Crit. Care Med. 1999;27:1230–1251. - PubMed
-
- Lavrentieva A, Kontakiotis T, Lazaridis L, Tsotsolis N, Koumis J, Kyriazis G, Bitzani M. Inflammatory markers in patients with severe burn injury. What is the best indicator of sepsis? Burns. 2007;33(2):189–94. - PubMed
-
- Bandyopadhyay G, De A, Laudanski K, Li F, Lentz C, Bankey P, Miller-Graziano C. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35(3):794–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

