Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system
- PMID: 17895971
- PMCID: PMC1976556
- DOI: 10.1371/journal.pone.0000930
Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system
Abstract
Background: Alcohol dependence and associated cognitive impairments apparently result from neuroadaptations to chronic alcohol consumption involving changes in expression of multiple genes. Here we investigated whether transcription factors of Nuclear Factor-kappaB (NF-kappaB) family, controlling neuronal plasticity and neurodegeneration, are involved in these adaptations in human chronic alcoholics.
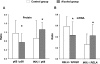
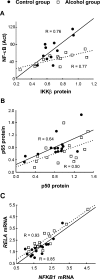
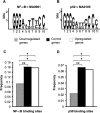
Methods and findings: Analysis of DNA-binding of NF-kappaB (p65/p50 heterodimer) and the p50 homodimer as well as NF-kappaB proteins and mRNAs was performed in postmortem human brain samples from 15 chronic alcoholics and 15 control subjects. The prefrontal cortex involved in alcohol dependence and cognition was analyzed and the motor cortex was studied for comparison. The p50 homodimer was identified as dominant kappaB binding factor in analyzed tissues. NF-kappaB and p50 homodimer DNA-binding was downregulated, levels of p65 (RELA) mRNA were attenuated, and the stoichiometry of p65/p50 proteins and respective mRNAs was altered in the prefrontal cortex of alcoholics. Comparison of a number of p50 homodimer/NF-kappaB target DNA sites, kappaB elements in 479 genes, down- or upregulated in alcoholics demonstrated that genes with kappaB elements were generally upregulated in alcoholics. No significant differences between alcoholics and controls were observed in the motor cortex.
Conclusions: We suggest that cycles of alcohol intoxication/withdrawal, which may initially activate NF-kappaB, when repeated over years downregulate RELA expression and NF-kappaB and p50 homodimer DNA-binding. Downregulation of the dominant p50 homodimer, a potent inhibitor of gene transcription apparently resulted in derepression of kappaB regulated genes. Alterations in expression of p50 homodimer/NF-kappaB regulated genes may contribute to neuroplastic adaptation underlying alcoholism.
Conflict of interest statement
Figures



References
-
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. - PubMed
-
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. - PubMed
-
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Neurol Clin. 1993;11:205–218. - PubMed
-
- Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, et al. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord. 2005;20:286–291. - PubMed
-
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, et al. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29:1504–1513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

