Constitutive phosphorylation of aurora-a on ser51 induces its stabilization and consequent overexpression in cancer
- PMID: 17895985
- PMCID: PMC1976594
- DOI: 10.1371/journal.pone.0000944
Constitutive phosphorylation of aurora-a on ser51 induces its stabilization and consequent overexpression in cancer
Abstract
Background: The serine/threonine kinase Aurora-A (Aur-A) is a proto-oncoprotein overexpressed in a wide range of human cancers. Overexpression of Aur-A is thought to be caused by gene amplification or mRNA overexpression. However, recent evidence revealed that the discrepancies between amplification of Aur-A and overexpression rates of Aur-A mRNA were observed in breast cancer, gastric cancer, hepatocellular carcinoma, and ovarian cancer. We found that aggressive head and neck cancers exhibited overexpression and stabilization of Aur-A protein without gene amplification or mRNA overexpression. Here we tested the hypothesis that aberration of the protein destruction system induces accumulation and consequently overexpression of Aur-A in cancer.
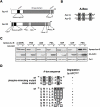
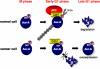
Principal findings: Aur-A protein was ubiquitinylated by APC(Cdh1) and consequently degraded when cells exited mitosis, and phosphorylation of Aur-A on Ser51 was observed during mitosis. Phosphorylation of Aur-A on Ser51 inhibited its APC(Cdh1)-mediated ubiquitylation and consequent degradation. Interestingly, constitutive phosphorylation on Ser51 was observed in head and neck cancer cells with protein overexpression and stabilization. Indeed, phosphorylation on Ser51 was observed in head and neck cancer tissues with Aur-A protein overexpression. Moreover, an Aur-A Ser51 phospho-mimetic mutant displayed stabilization of protein during cell cycle progression and enhanced ability to cell transformation.
Conclusions/significance: Broadly, this study identifies a new mode of Aur-A overexpression in cancer through phosphorylation-dependent inhibition of its proteolysis in addition to gene amplification and mRNA overexpression. We suggest that the inhibition of Aur-A phosphorylation can represent a novel way to decrease Aur-A levels in cancer therapy.
Conflict of interest statement
Figures





References
-
- Murray AW. Recycling the Cell Cycle: Cyclins Revisited. Cell. 2004;116:221–234. - PubMed
-
- Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. - PubMed
-
- Marumoto T, Zhang D, Saya H. Aurora-A-a Guardian of poles. Nat Rev Cancer. 2005;5:42–50. - PubMed
-
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

