Distinct roles of Hoxa2 and Krox20 in the development of rhythmic neural networks controlling inspiratory depth, respiratory frequency, and jaw opening
- PMID: 17897445
- PMCID: PMC2098766
- DOI: 10.1186/1749-8104-2-19
Distinct roles of Hoxa2 and Krox20 in the development of rhythmic neural networks controlling inspiratory depth, respiratory frequency, and jaw opening
Abstract
Background: Little is known about the involvement of molecular determinants of segmental patterning of rhombomeres (r) in the development of rhythmic neural networks in the mouse hindbrain. Here, we compare the phenotypes of mice carrying targeted inactivations of Hoxa2, the only Hox gene expressed up to r2, and of Krox20, expressed in r3 and r5. We investigated the impact of such mutations on the neural circuits controlling jaw opening and breathing in newborn mice, compatible with Hoxa2-dependent trigeminal defects and direct regulation of Hoxa2 by Krox20 in r3.
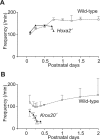
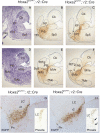
Results: We found that Hoxa2 mutants displayed an impaired oro-buccal reflex, similarly to Krox20 mutants. In contrast, while Krox20 is required for the development of the rhythm-promoting parafacial respiratory group (pFRG) modulating respiratory frequency, Hoxa2 inactivation did not affect neonatal breathing frequency. Instead, we found that Hoxa2-/- but not Krox20-/- mutation leads to the elimination of a transient control of the inspiratory amplitude normally occurring during the first hours following birth. Tracing of r2-specific progenies of Hoxa2 expressing cells indicated that the control of inspiratory activity resides in rostral pontine areas and required an intact r2-derived territory.
Conclusion: Thus, inspiratory shaping and respiratory frequency are under the control of distinct Hox-dependent segmental cues in the mammalian brain. Moreover, these data point to the importance of rhombomere-specific genetic control in the development of modular neural networks in the mammalian hindbrain.
Figures


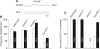
Similar articles
-
Rostral hindbrain patterning involves the direct activation of a Krox20 transcriptional enhancer by Hox/Pbx and Meis factors.Development. 2008 Oct;135(20):3369-78. doi: 10.1242/dev.023614. Epub 2008 Sep 11. Development. 2008. PMID: 18787068
-
Different respiratory control systems are affected in homozygous and heterozygous kreisler mutant mice.Eur J Neurosci. 2002 Feb;15(4):684-92. doi: 10.1046/j.1460-9568.2002.01909.x. Eur J Neurosci. 2002. PMID: 11886449
-
Differences in Krox20-dependent regulation of Hoxa2 and Hoxb2 during hindbrain development.Dev Biol. 2001 May 15;233(2):468-81. doi: 10.1006/dbio.2001.0197. Dev Biol. 2001. PMID: 11336508
-
Conservation and diversity in the cis-regulatory networks that integrate information controlling expression of Hoxa2 in hindbrain and cranial neural crest cells in vertebrates.Dev Biol. 2002 Jun 1;246(1):45-56. doi: 10.1006/dbio.2002.0665. Dev Biol. 2002. PMID: 12027433 Review.
-
Developmental basis of the rostro-caudal organization of the brainstem respiratory rhythm generator.Philos Trans R Soc Lond B Biol Sci. 2009 Sep 12;364(1529):2469-76. doi: 10.1098/rstb.2009.0090. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 19651648 Free PMC article. Review.
Cited by
-
Molecular Segmentation of the Spinal Trigeminal Nucleus in the Adult Mouse Brain.Front Neuroanat. 2021 Dec 10;15:785840. doi: 10.3389/fnana.2021.785840. eCollection 2021. Front Neuroanat. 2021. PMID: 34955765 Free PMC article.
-
Ablation of Zfhx4 results in early postnatal lethality by disrupting the respiratory center in mice.J Mol Cell Biol. 2021 Jul 6;13(3):210-224. doi: 10.1093/jmcb/mjaa081. J Mol Cell Biol. 2021. PMID: 33475140 Free PMC article.
-
Irregular Breathing in Mice following Genetic Ablation of V2a Neurons.J Neurosci. 2012 Jun 6;32(23):7895-906. doi: 10.1523/JNEUROSCI.0445-12.2012. J Neurosci. 2012. PMID: 22674265 Free PMC article.
-
Hox genes: choreographers in neural development, architects of circuit organization.Neuron. 2013 Oct 2;80(1):12-34. doi: 10.1016/j.neuron.2013.09.020. Epub 2013 Oct 2. Neuron. 2013. PMID: 24094100 Free PMC article. Review.
-
The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO₂.Elife. 2015 Apr 13;4:e07051. doi: 10.7554/eLife.07051. Elife. 2015. PMID: 25866925 Free PMC article.
References
-
- Rijli FM, Gavalas A, Chambon P. Segmentation and specification in the branchial region of the head: the role of the Hox selector genes. Int J Dev Biol. 1998;42:393–401. - PubMed
-
- Nonchev S, Vesque C, Maconochie M, Seitanidou T, Ariza-McNaughton L, Frain M, Marshall H, Sham MH, Krumlauf R, Charnay P. Segmental expression of Hoxa-2 in the hindbrain is directly regulated by Krox20 . Development. 1996;122:543–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

