Dynamic remodelling of synapses can occur in the absence of the parent cell body
- PMID: 17897464
- PMCID: PMC2048966
- DOI: 10.1186/1471-2202-8-79
Dynamic remodelling of synapses can occur in the absence of the parent cell body
Abstract
Background: Retraction of nerve terminals is a characteristic feature of development, injury and insult and may herald many neurodegenerative diseases. Although morphological events have been well characterized, we know relatively little about the nature of the underlying cellular machinery. Evidence suggests a strong local component in determining which neuronal branches and synapses are lost, but a greater understanding of this basic neurological process is required. Here we test the hypothesis that nerve terminals are semi-autonomous and able to rapidly respond to local stimuli in the absence of communication with their parent cell body.
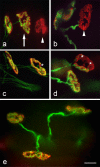
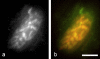
Results: We used an isolated preparation consisting of distal peripheral nerve stumps, associated nerve terminals and post-synaptic muscle fibres, maintained in-vitro for up to 3 hrs. In this system synapses are intact but the presynaptic nerve terminal is disconnected from its cell soma. In control preparations synapses were stable for extended periods and did not undergo Wallerian degeneration. In contrast, addition of purines triggers rapid changes at synapses. Using fluorescence and electron microscopy we observe ultrastructural and gross morphological events consistent with nerve terminal retraction. We find no evidence of Wallerian or Wallerian-like degeneration in these preparations. Pharmacological experiments implicate pre-synaptic P2X7 receptor subunits as key mediators of these events.
Conclusion: The data presented suggest; first that isolated nerve terminals are able to regulate connectivity independent of signals from the cell body, second that synapses exist in a dynamic state, poised to shift from stability to loss by activating intrinsic mechanisms and molecules, and third that local purines acting at purinergic receptors can trigger these events. A role for ATP receptors in this is not surprising since they are frequently activated during cellular injury, when adenosine tri-phosphate is released from damaged cells. Local control demands that the elements necessary to drive retraction are constitutively present. We hypothesize that pre-existing scaffolds of molecular motors and cytoskeletal proteins could provide the dynamism required to drive such structural changes in nerve terminals in the absence of the cell body.
Figures




References
-
- Lohof AM, Delhayebouchaud N, Mariani J. Synapse Elimination In the Central-Nervous-System – Functional- Significance and Cellular Mechanisms. Rev Neurosci. 1996;7:85–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

