A pilot study for augmenting atomoxetine with methylphenidate: safety of concomitant therapy in children with attention-deficit/hyperactivity disorder
- PMID: 17897473
- PMCID: PMC2098748
- DOI: 10.1186/1753-2000-1-10
A pilot study for augmenting atomoxetine with methylphenidate: safety of concomitant therapy in children with attention-deficit/hyperactivity disorder
Abstract
Background: This study examined augmenting atomoxetine with extended-release methylphenidate in children whose attention-deficit/hyperactivity disorder (ADHD) previously failed to respond adequately to stimulant medication.
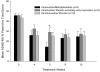
Methods: Children with ADHD and prior stimulant treatment (N = 25) received atomoxetine (1.2 mg/kg/day) plus placebo. After 4 weeks, patients who were responders (n = 4) were continued on atomoxetine/placebo while remaining patients were randomly assigned to either methylphenidate (ATX/MPH) (1.1 mg/kg/day) or placebo augmentation (ATX/PB) for another 6 weeks. Patients and sites were blind to timing of active augmentation. Safety measures included vital signs, weight, and adverse events. Efficacy was assessed by ADHD rating scales.
Results: Categorical increases in vital signs occurred for 5 patients (3 patients in ATX/MPH, 2 patients in ATX/PBO). Sixteen percent discontinued the study due to AE, but no difference between augmentation groups. Atomoxetine treatment was efficacious on outcome measures (P <or= .001), but methylphenidate did not enhance response.
Conclusion: Methylphenidate appears to be safely combined with atomoxetine, but conclusions limited by small sample. With atomoxetine treatment, 43% of patients achieved normalization on ADHD ratings.
Figures
References
-
- Carlson GA, Rapport MD, Kelly K, Grayson P, Pataki CS. Methylphenidate and desipramine in hospitalized children with comorbid behavior and mood disorders: separate and combined effects on behavior and mood. J Child Adolesc Psychopharmacol. 1995;5:191–204.
LinkOut - more resources
Full Text Sources