A global analysis of genetic interactions in Caenorhabditis elegans
- PMID: 17897480
- PMCID: PMC2373897
- DOI: 10.1186/jbiol58
A global analysis of genetic interactions in Caenorhabditis elegans
Abstract
Background: Understanding gene function and genetic relationships is fundamental to our efforts to better understand biological systems. Previous studies systematically describing genetic interactions on a global scale have either focused on core biological processes in protozoans or surveyed catastrophic interactions in metazoans. Here, we describe a reliable high-throughput approach capable of revealing both weak and strong genetic interactions in the nematode Caenorhabditis elegans.
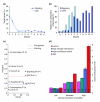
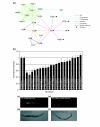
Results: We investigated interactions between 11 'query' mutants in conserved signal transduction pathways and hundreds of 'target' genes compromised by RNA interference (RNAi). Mutant-RNAi combinations that grew more slowly than controls were identified, and genetic interactions inferred through an unbiased global analysis of the interaction matrix. A network of 1,246 interactions was uncovered, establishing the largest metazoan genetic-interaction network to date. We refer to this approach as systematic genetic interaction analysis (SGI). To investigate how genetic interactions connect genes on a global scale, we superimposed the SGI network on existing networks of physical, genetic, phenotypic and coexpression interactions. We identified 56 putative functional modules within the superimposed network, one of which regulates fat accumulation and is coordinated by interactions with bar-1(ga80), which encodes a homolog of beta-catenin. We also discovered that SGI interactions link distinct subnetworks on a global scale. Finally, we showed that the properties of genetic networks are conserved between C. elegans and Saccharomyces cerevisiae, but that the connectivity of interactions within the current networks is not.
Conclusions: Synthetic genetic interactions may reveal redundancy among functional modules on a global scale, which is a previously unappreciated level of organization within metazoan systems. Although the buffering between functional modules may differ between species, studying these differences may provide insight into the evolution of divergent form and function.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

