Ubiquitylation of proteins in livers of hibernating golden-mantled ground squirrels, Spermophilus lateralis
- PMID: 17897639
- PMCID: PMC2700031
- DOI: 10.1016/j.cryobiol.2007.08.003
Ubiquitylation of proteins in livers of hibernating golden-mantled ground squirrels, Spermophilus lateralis
Abstract
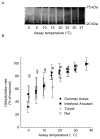
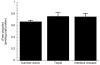
Rodent hibernators experience low core body temperature (as low as -2 degrees C) and reduced metabolic rates during hibernation. Concordant with energetic constraints, protein synthesis is negligible during torpor. To maintain pools of key regulatory proteins, proteolysis must be depressed as well. Ubiquitin-dependent proteolysis consists of two major steps: (1) ubiquitylation or tagging of a protein substrate by ubiquitin and (2) the protein substrate's subsequent degradation by the 26S proteasome. Earlier, we demonstrated that the low temperatures typical of torpor virtually arrest proteolytic processing. Here, we demonstrate that in vitro ubiquitylation still continues at greater than 30% of maximal rates at temperatures as low as 0 degrees C. Continued ubiquitylation in the presence of severely depressed proteolysis may explain the previously observed 2- to 3-fold increase of ubiquitin conjugates during torpor. We determined if there is a qualitative change in the type of ubiquitylation e.g., monoubiquitylation vs polyubiquitylation that occurs during torpor. We found no bias for monoubiquitylation in any state of the torpor cycle. We further determined that substrate limitation of free ubiquitin is not limiting ubiquitylation during torpor. We conclude that while the cold temperatures of torpor may limit proteolysis in accordance with metabolic demands, continued ubiquitylation may result in increased ubiquitin conjugate concentrations that must be processed upon arousal.
Figures
Similar articles
-
Ubiquitin conjugate dynamics in the gut and liver of hibernating ground squirrels.J Comp Physiol B. 2002 Apr;172(3):269-73. doi: 10.1007/s00360-002-0252-5. Epub 2002 Feb 20. J Comp Physiol B. 2002. PMID: 11919708
-
Life in the slow lane: low rates of ubiquitin-dependent proteolysis in the heterothermic and heterometabolic tenrec, Tenrec ecaudatus.J Comp Physiol B. 2025 Jun;195(3):405-413. doi: 10.1007/s00360-025-01624-1. Epub 2025 Jun 10. J Comp Physiol B. 2025. PMID: 40493084
-
Proteolysis is depressed during torpor in hibernators at the level of the 20S core protease.J Comp Physiol B. 2005 Jul;175(5):329-35. doi: 10.1007/s00360-005-0489-x. Epub 2005 May 24. J Comp Physiol B. 2005. PMID: 15912363
-
Neuroprotection: lessons from hibernators.Comp Biochem Physiol B Biochem Mol Biol. 2012 May;162(1-3):1-9. doi: 10.1016/j.cbpb.2012.01.008. Epub 2012 Feb 3. Comp Biochem Physiol B Biochem Mol Biol. 2012. PMID: 22326449 Free PMC article. Review.
-
Protein ubiquitylation and synaptic function.Ann N Y Acad Sci. 2003 Sep;998:33-40. doi: 10.1196/annals.1254.006. Ann N Y Acad Sci. 2003. PMID: 14592861 Review.
Cited by
-
Activity, abundance and expression of Ca²⁺-activated proteases in skeletal muscle of the aestivating frog, Cyclorana alboguttata.J Comp Physiol B. 2015 Feb;185(2):243-55. doi: 10.1007/s00360-014-0880-6. Epub 2014 Dec 12. J Comp Physiol B. 2015. PMID: 25502658
-
Muscle plasticity in hibernating ground squirrels (Spermophilus lateralis) is induced by seasonal, but not low-temperature, mechanisms.J Comp Physiol B. 2011 Jan;181(1):147-64. doi: 10.1007/s00360-010-0505-7. Epub 2010 Aug 12. J Comp Physiol B. 2011. PMID: 20703484
-
Applying systems-level approaches to elucidate regulatory function during mammalian hibernation.Temperature (Austin). 2016 May 4;3(4):524-526. doi: 10.1080/23328940.2016.1182243. eCollection 2016. Temperature (Austin). 2016. PMID: 28090555 Free PMC article. No abstract available.
-
Patterns of ubiquitylation and SUMOylation associated with exposure to anoxia in embryos of the annual killifish Austrofundulus limnaeus.J Comp Physiol B. 2014 Feb;184(2):235-47. doi: 10.1007/s00360-013-0791-y. Epub 2013 Dec 14. J Comp Physiol B. 2014. PMID: 24337451 Free PMC article.
-
Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion.Stroke. 2012 Aug;43(8):2229-35. doi: 10.1161/STROKEAHA.112.650416. Epub 2012 Jun 14. Stroke. 2012. PMID: 22700531 Free PMC article.
References
-
- Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695:189–207. - PubMed
-
- Anchordoguy TJ, Hand SC. Acute blockage of the ubiquitin-mediated proteolytic pathway during invertebrate quiescence. Am J Physiol. 1994;267:R895–900. - PubMed
-
- Barnes BM. Freeze avoidance in a mammal: body temperature below 0° C in an Arctic hibernator. Science. 1989;244:1593–1595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

