Proteomic analysis of ovomucoid hypersensitivity in mice by two-dimensional difference gel electrophoresis (2D-DIGE)
- PMID: 17897766
- PMCID: PMC7126535
- DOI: 10.1016/j.fct.2007.06.039
Proteomic analysis of ovomucoid hypersensitivity in mice by two-dimensional difference gel electrophoresis (2D-DIGE)
Abstract
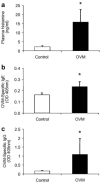
There is a need to develop reliable methods to assess the safety of genetically modified and other novel foods. The aim of this study was to identify protein biomarkers of food allergy in mice exposed to ovomucoid (OVM), a major food allergen found in chicken egg white. BALB/c mice were repeatedly sensitized by gavage with OVM and cholera toxin (CT) and control mice were exposed to a mixture of amino acids with CT. At the endpoint, all mice were challenged intraperitoneally with OVM and alum. Type-1 hypersensitivity was confirmed in OVM-sensitized mice by observation of clinical signs of anaphylaxis and elevated levels of plasma histamine, OVM-specific IgE and OVM-specific IgG by ELISA. Differential protein expression was assessed in albumin-depleted plasma as well as in mesenteric lymph node, liver, spleen, and ileum by two-dimensional difference gel electrophoresis (2D-DIGE). Differentially expressed proteins were identified by liquid chromatography with tandem mass spectrometry. Plasma proteins overexpressed in OVM-sensitized mice included haptoglobin (41-fold), serum amyloid A (19-fold) and peroxiredoxin-2 (1.9-fold). Further validation of these plasma proteins in other animal models of food allergy with different food allergens is required to assess their potential as candidate biomarkers for use in evaluating the allergenicity of novel foods.
Figures


Similar articles
-
A neonatal swine model of allergy induced by the major food allergen chicken ovomucoid (Gal d 1).Int Arch Allergy Immunol. 2008;146(1):11-8. doi: 10.1159/000112498. Epub 2007 Dec 14. Int Arch Allergy Immunol. 2008. PMID: 18087157
-
Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy.J Allergy Clin Immunol. 2012 Mar;129(3):739-47. doi: 10.1016/j.jaci.2011.11.053. Epub 2012 Jan 24. J Allergy Clin Immunol. 2012. PMID: 22277199
-
Protease-digested egg-white products induce oral tolerance in mice but elicit little IgE production upon epicutaneous exposure.Allergol Int. 2022 Oct;71(4):528-535. doi: 10.1016/j.alit.2022.03.006. Epub 2022 Apr 18. Allergol Int. 2022. PMID: 35443911
-
Molecular diagnosis of egg allergy.Curr Opin Allergy Clin Immunol. 2011 Jun;11(3):210-5. doi: 10.1097/ACI.0b013e3283464d1b. Curr Opin Allergy Clin Immunol. 2011. PMID: 21467927 Review.
-
Molecular diagnosis of egg allergy: an update.Expert Rev Mol Diagn. 2015;15(7):895-906. doi: 10.1586/14737159.2015.1041927. Epub 2015 May 15. Expert Rev Mol Diagn. 2015. PMID: 25975845 Review.
Cited by
-
Proteomics Study on Nonallergic Hypersensitivity Induced by Compound 4880 and Ovalbumin.PLoS One. 2016 Feb 1;11(2):e0148262. doi: 10.1371/journal.pone.0148262. eCollection 2016. PLoS One. 2016. PMID: 26829397 Free PMC article.
-
The day/night proteome in the murine heart.Am J Physiol Regul Integr Comp Physiol. 2014 Jul 15;307(2):R121-37. doi: 10.1152/ajpregu.00011.2014. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24789993 Free PMC article.
-
A systematic review and meta-analysis of proteomic and metabolomic alterations in anaphylaxis reactions.Front Immunol. 2024 Feb 7;15:1328212. doi: 10.3389/fimmu.2024.1328212. eCollection 2024. Front Immunol. 2024. PMID: 38384462 Free PMC article.
References
-
- Adel-Patient K., Bernard H., Ah-Leung S., Creminon C., Wal J.M. Peanut- and cow’s milk-specific IgE, Th2 cells and local anaphylactic reaction are induced in BALB/c mice orally sensitized with cholera toxin. Allergy. 2005;60:658–664. - PubMed
-
- Alban A., David S.O., Bjorkesten L., Andersson C., Sloge E., Lewis S., Currie I. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. - PubMed
-
- Baseler M.W., Burrell R. Purification of haptoglobin and its effects on lymphocyte and alveolar macrophage responses. Inflammation. 1983;7:387–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

