HC-Pro protein of Potato virus Y can interact with three Arabidopsis 20S proteasome subunits in planta
- PMID: 17898064
- PMCID: PMC2169110
- DOI: 10.1128/JVI.00913-07
HC-Pro protein of Potato virus Y can interact with three Arabidopsis 20S proteasome subunits in planta
Abstract
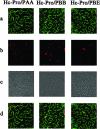
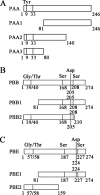
The multifunctional protein helper component proteinase (HC-Pro) is thought to interfere with the activity of the 20S proteasome; however, no sites of interaction have been identified for either protein. Here, we first show that the Potato virus Y (PVY) HC-Pro protein can interact with three Arabidopsis 20S proteasome subunits (PAA, PBB, and PBE), using a yeast two-hybrid system and the bimolecular fluorescence complement assay. In addition, yeast two-hybrid analysis of the interaction between several mutant subunits of the 20S proteasome and PVY HC-Pro confirmed that residues 81 to 140 of PAA, 1 to 80 of PBB, and 160 to 274 of PBE are necessary for binding PAA, PBB, and PBE to PVY HC-Pro, respectively. Deletion mutant analysis of PVY HC-Pro showed that the N terminus (residues 1 to 97) is necessary for its interaction with three Arabidopsis 20S proteasome subunits. The ability of HC-Pro to interact and interfere with the activity of the 20S proteasome may help explain the molecular basis of its multifunctional character.
Figures





References
-
- Apcher, G. S., S. Heink, D. Zantopf, P. M. Kloetzel, H. P. Schmid, R. J. Mayer, and E. Kruger. 2003. Human immunodeficiency virus-1 Tat protein interacts with distinct proteasomal alpha and beta subunits. FEBS Lett. 553:200-204. - PubMed
-
- Aviel, S., G. Winberg, M. Massucci, and A. Ciechanover. 2000. Degradation of the Epstein-Barr virus latent membrane protein 1 (LMP1) by the ubiquitin-proteasome pathway. Targeting via ubiquitination of the N-terminal residue. J. Biol. Chem. 275:23491-23499. - PubMed
-
- Ballut, L., M. Drucker, M. Pugniere, F. Cambon, S. Blanc, F. Roquet, T. Candresse, H. P. Schmid, P. Nicolas, O. L. Gall, and S. Badaoui. 2005. HcPro, a multifunctional protein encoded by a plant RNA virus, targets the 20S proteasome and affects its enzymic activities. J. Gen. Virol. 86:2595-2603. - PubMed
-
- Ballut, L., F. Petit, S. Mouzeyar, O. Le Gall, T. Candresse, P. Schmid, P. Nicolas, and S. Badaoui. 2003. Biochemical identification of proteasome-associated endonuclease activity in sunflower. Biochim. Biophys. Acta 1645:30-39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

