Crimean-Congo hemorrhagic fever virus glycoprotein processing by the endoprotease SKI-1/S1P is critical for virus infectivity
- PMID: 17898072
- PMCID: PMC2169102
- DOI: 10.1128/JVI.01647-07
Crimean-Congo hemorrhagic fever virus glycoprotein processing by the endoprotease SKI-1/S1P is critical for virus infectivity
Abstract
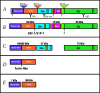
Crimean-Congo hemorrhagic fever virus (CCHFV) causes severe human disease. The CCHFV medium RNA encodes a polyprotein which is proteolytically processed to yield the glycoprotein precursors PreGn and PreGc, followed by structural glycoproteins Gn and Gc. Subtilisin kexin isozyme-1/site-1 protease (SKI-1/S1P) plays a central role in Gn processing. Here we show that CCHFV-infected cells deficient in SKI-1/S1P produce no infectious virus, although PreGn and PreGc accumulated normally in the Golgi apparatus, the site of virus assembly. Only nucleoprotein-containing particles which lacked virus glycoproteins (Gn/Gc or PreGn/PreGc) were secreted. Complementation of SKI-1/S1P-deficient cells with a SKI-1/S1P expression vector restored release of infectious virus (>10(6) PFU/ml), confirming that SKI-1/S1P processing is required for incorporation of viral glycoproteins. SKI-1/S1P may represent a promising antiviral target.
Figures

Similar articles
-
Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin.PLoS Pathog. 2015 May 1;11(5):e1004879. doi: 10.1371/journal.ppat.1004879. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25933376 Free PMC article.
-
The proprotein convertase SKI-1/S1P is a critical host factor for Nairobi sheep disease virus infectivity.Virus Res. 2023 May;329:199099. doi: 10.1016/j.virusres.2023.199099. Epub 2023 Mar 22. Virus Res. 2023. PMID: 36948228 Free PMC article.
-
Crimean-congo hemorrhagic fever virus glycoprotein precursor is cleaved by Furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein.J Virol. 2006 Jan;80(1):514-25. doi: 10.1128/JVI.80.1.514-525.2006. J Virol. 2006. PMID: 16352575 Free PMC article.
-
How Do Enveloped Viruses Exploit the Secretory Proprotein Convertases to Regulate Infectivity and Spread?Viruses. 2021 Jun 25;13(7):1229. doi: 10.3390/v13071229. Viruses. 2021. PMID: 34202098 Free PMC article. Review.
-
Envelope glycoprotein of arenaviruses.Viruses. 2012 Oct 17;4(10):2162-81. doi: 10.3390/v4102162. Viruses. 2012. PMID: 23202458 Free PMC article. Review.
Cited by
-
Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin.PLoS Pathog. 2015 May 1;11(5):e1004879. doi: 10.1371/journal.ppat.1004879. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25933376 Free PMC article.
-
Transstadial Transmission and Long-term Association of Crimean-Congo Hemorrhagic Fever Virus in Ticks Shapes Genome Plasticity.Sci Rep. 2016 Oct 24;6:35819. doi: 10.1038/srep35819. Sci Rep. 2016. PMID: 27775001 Free PMC article.
-
Interferon and cytokine responses to Crimean Congo hemorrhagic fever virus; an emerging and neglected viral zonoosis.Cytokine Growth Factor Rev. 2008 Oct-Dec;19(5-6):395-404. doi: 10.1016/j.cytogfr.2008.11.001. Epub 2008 Nov 21. Cytokine Growth Factor Rev. 2008. PMID: 19027345 Free PMC article. Review.
-
Site-1 protease inhibits mitochondrial respiration by controlling the TGF-β target gene Mss51.Cell Rep. 2023 Apr 25;42(4):112336. doi: 10.1016/j.celrep.2023.112336. Epub 2023 Mar 31. Cell Rep. 2023. PMID: 37002920 Free PMC article.
-
Antibodies targeting Crimean-Congo hemorrhagic fever virus GP38 limit vascular leak and viral spread.Sci Transl Med. 2025 Feb 19;17(786):eadq5928. doi: 10.1126/scitranslmed.adq5928. Epub 2025 Feb 19. Sci Transl Med. 2025. PMID: 39970234 Free PMC article.
References
-
- Elshuber, S., S. L. Allison, F. X. Heinz, and C. W. Mandl. 2003. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J. Gen. Virol. 84:183-191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

