Cytoplasmic relocalization of heterogeneous nuclear ribonucleoprotein A1 controls translation initiation of specific mRNAs
- PMID: 17898077
- PMCID: PMC2096577
- DOI: 10.1091/mbc.e07-06-0603
Cytoplasmic relocalization of heterogeneous nuclear ribonucleoprotein A1 controls translation initiation of specific mRNAs
Abstract
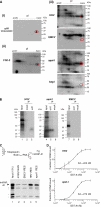
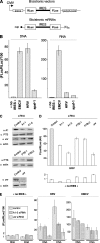
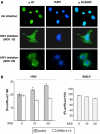
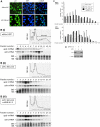
Heterogeneous nuclear ribonucleoprotein (hnRNP) A1 is a nucleocytoplasmic shuttling protein that regulates gene expression through its action on mRNA metabolism and translation. The cytoplasmic redistribution of hnRNP A1 is a regulated process during viral infection and cellular stress. Here, we show that hnRNP A1 is an internal ribosome entry site (IRES) trans-acting factor that binds specifically to the 5' untranslated region of both the human rhinovirus-2 and the human apoptotic peptidase activating factor 1 (apaf-1) mRNAs, thereby regulating their translation. Furthermore, the cytoplasmic redistribution of hnRNP A1 after rhinovirus infection leads to enhanced rhinovirus IRES-mediated translation, whereas the cytoplasmic relocalization of hnRNP A1 after UVC irradiation limits the UVC-triggered translational activation of the apaf-1 IRES. Therefore, this study provides a direct demonstration that IRESs behave as translational enhancer elements regulated by specific trans-acting mRNA binding proteins in given physiological conditions. Our data highlight a new way to regulate protein synthesis in eukaryotes through the subcellular relocalization of a nuclear mRNA-binding protein.
Figures


Similar articles
-
hnRNP A1 interacts with the 5' untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication.J Virol. 2009 Jun;83(12):6106-14. doi: 10.1128/JVI.02476-08. Epub 2009 Apr 1. J Virol. 2009. PMID: 19339352 Free PMC article.
-
Subcellular relocalization of a trans-acting factor regulates XIAP IRES-dependent translation.Mol Biol Cell. 2007 Apr;18(4):1302-11. doi: 10.1091/mbc.e06-06-0515. Epub 2007 Feb 7. Mol Biol Cell. 2007. PMID: 17287399 Free PMC article.
-
hnRNP A1 mediates the activation of the IRES-dependent SREBP-1a mRNA translation in response to endoplasmic reticulum stress.Biochem J. 2013 Jan 15;449(2):543-53. doi: 10.1042/BJ20120906. Biochem J. 2013. PMID: 23106379
-
IRES Trans-Acting Factors, Key Actors of the Stress Response.Int J Mol Sci. 2019 Feb 20;20(4):924. doi: 10.3390/ijms20040924. Int J Mol Sci. 2019. PMID: 30791615 Free PMC article. Review.
-
Heterogeneous nuclear ribonucleoprotein A1 in health and neurodegenerative disease: from structural insights to post-transcriptional regulatory roles.Mol Cell Neurosci. 2013 Sep;56:436-46. doi: 10.1016/j.mcn.2012.12.002. Epub 2012 Dec 14. Mol Cell Neurosci. 2013. PMID: 23247072 Review.
Cited by
-
Downregulation of Nipah virus N mRNA occurs through interaction between its 3' untranslated region and hnRNP D.J Virol. 2013 Jun;87(12):6582-8. doi: 10.1128/JVI.02495-12. Epub 2013 Mar 20. J Virol. 2013. PMID: 23514888 Free PMC article.
-
Insights into the biology of IRES elements through riboproteomic approaches.J Biomed Biotechnol. 2010;2010:458927. doi: 10.1155/2010/458927. Epub 2010 Feb 2. J Biomed Biotechnol. 2010. PMID: 20150968 Free PMC article. Review.
-
N protein of PEDV plays chess game with host proteins by selective autophagy.Autophagy. 2023 Aug;19(8):2338-2352. doi: 10.1080/15548627.2023.2181615. Epub 2023 Mar 2. Autophagy. 2023. PMID: 36861818 Free PMC article.
-
Complex interactomes and post-translational modifications of the regulatory proteins HABP4 and SERBP1 suggest pleiotropic cellular functions.World J Biol Chem. 2019 Nov 21;10(3):44-64. doi: 10.4331/wjbc.v10.i3.44. World J Biol Chem. 2019. PMID: 31768228 Free PMC article. Review.
-
RNA-Binding Proteins in Amyotrophic Lateral Sclerosis.Mol Cells. 2018 Sep 30;41(9):818-829. doi: 10.14348/molcells.2018.0243. Epub 2018 Aug 29. Mol Cells. 2018. PMID: 30157547 Free PMC article. Review.
References
-
- Bonnal S., Pileur F., Orsini C., Parker F., Pujol F., Prats A. C., Vagner S. Heterogeneous nuclear ribonucleoprotein A1 is a novel internal ribosome entry site trans-acting factor that modulates alternative initiation of translation of the fibroblast growth factor 2 mRNA. J. Biol. Chem. 2005;280:4144–4153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

