Attention improves population-level frequency tuning in human auditory cortex
- PMID: 17898210
- PMCID: PMC6673146
- DOI: 10.1523/JNEUROSCI.2963-07.2007
Attention improves population-level frequency tuning in human auditory cortex
Abstract
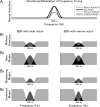
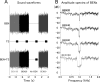
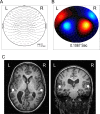
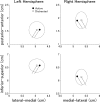
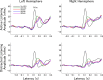
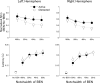
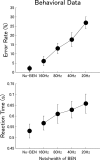
Attention improves auditory performance in noisy environments by either enhancing the processing of task-relevant stimuli ("gain"), suppressing task-irrelevant information ("sharpening"), or both. In the present study, we investigated the effect of focused auditory attention on the population-level frequency tuning in human auditory cortex by means of magnetoencephalography. Using complex stimuli consisting of a test tone superimposed on different band-eliminated noises during active listening or distracted listening conditions, we observed that focused auditory attention caused not only gain, but also sharpening of frequency tuning in human auditory cortex as reflected by the N1m auditory evoked response. This combination of gain and sharpening in the auditory cortex may contribute to better auditory performance during focused auditory attention.
Figures
References
-
- Alain C, Arnott SR. Selectively attending to auditory objects. Front Biosci. 2000;5:D202–D212. - PubMed
-
- Barbour DL, Wang X. Temporal coherence sensitivity in auditory cortex. J Neurophysiol. 2002;88:2684–2699. - PubMed
-
- Benedict RH, Lockwood AH, Shucard JL, Shucard DW, Wack D, Murphy BW. Functional neuroimaging of attention in the auditory modality. NeuroReport. 1998;9:121–126. - PubMed
-
- Boemio A, Fromm S, Braun A, Poeppel D. Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci. 2005;8:389–395. - PubMed
-
- Diamond DM, Weinberger NM. Physiological plasticity of single neurons in auditory cortex of the cat during acquisition of the pupillary conditioned response: II. Secondary field (AII) Behav Neurosci. 1984;98:189–210. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
