Rapid homeostatic plasticity in the intact adult visual system
- PMID: 17898227
- PMCID: PMC6673162
- DOI: 10.1523/JNEUROSCI.1631-07.2007
Rapid homeostatic plasticity in the intact adult visual system
Abstract
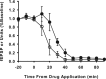
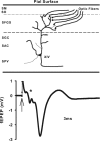
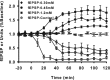
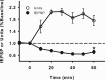
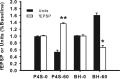
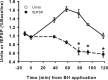
Neurons may possess activity-dependent homeostatic mechanisms that permit them to globally alter synaptic strength as activity varies. We used the retinotectal projection of goldfish to test this idea in the intact adult CNS. We first altered tectal neuron activity by selectively manipulating excitatory input. When excitatory synaptic drive to tectal neurons was eliminated by blocking optic fibers, current evoked at optic synapses increased by 183% within 90 min. With partial activity blockade, the increase in synaptic strength scaled with the magnitude of activity depression. This silence-induced potentiation was also rapidly reversible. Conversely, an increase in optic input was followed by a decrease in evoked synaptic current. When optic drive was not altered and tectal neuronal activity was instead increased or decreased pharmacologically via GABA(A) receptors, synaptic strength again changed inversely with activity, indicating that synaptic strength changed in response to neuronal activity and not excitatory drive. Furthermore, altered synaptic strength tended to return ongoing activity to baseline. Changes in synaptic strength could also be detected in heterosynaptic pathways, indicating a global response. Finally, changes in synaptic strength were associated with corresponding changes in ongoing and evoked firing rates, indicating that the responsivity of tectal neurons was altered. Thus, tectal neurons exhibit archetypical homeostasis, one of the first robust examples in the intact adult CNS.
Figures







Similar articles
-
Normal activity-dependent refinement in a compressed retinotectal projection in goldfish.J Comp Neurol. 1994 Sep 22;347(4):481-94. doi: 10.1002/cne.903470402. J Comp Neurol. 1994. PMID: 7529264
-
Apparent movement of optic terminals out of a local postsynaptically blocked region in goldfish optic tectum.J Neurophysiol. 1985 Jan;53(1):237-51. doi: 10.1152/jn.1985.53.1.237. J Neurophysiol. 1985. PMID: 2983036
-
Spontaneous bursting and long-lived local correlation in normal and denervated tectum of goldfish.J Neurobiol. 1995 Jan;26(1):109-18. doi: 10.1002/neu.480260109. J Neurobiol. 1995. PMID: 7714520
-
Mechanisms of homeostatic plasticity in the excitatory synapse.J Neurochem. 2016 Dec;139(6):973-996. doi: 10.1111/jnc.13687. Epub 2016 Jul 1. J Neurochem. 2016. PMID: 27241695 Review.
-
Consequences for retinal growth when vision occurs in S2 space.Biosystems. 1990;24(1):25-30. doi: 10.1016/0303-2647(90)90026-w. Biosystems. 1990. PMID: 2224067 Review.
Cited by
-
Can homeostatic plasticity in deafferented primary auditory cortex lead to travelling waves of excitation?J Comput Neurosci. 2011 Apr;30(2):279-99. doi: 10.1007/s10827-010-0256-1. Epub 2010 Jul 10. J Comput Neurosci. 2011. PMID: 20623168
-
Synaptic plasticity in neural networks needs homeostasis with a fast rate detector.PLoS Comput Biol. 2013;9(11):e1003330. doi: 10.1371/journal.pcbi.1003330. Epub 2013 Nov 14. PLoS Comput Biol. 2013. PMID: 24244138 Free PMC article.
-
The serum response factor and a putative novel transcription factor regulate expression of the immediate-early gene Arc/Arg3.1 in neurons.J Neurosci. 2009 Feb 4;29(5):1525-37. doi: 10.1523/JNEUROSCI.5575-08.2009. J Neurosci. 2009. PMID: 19193899 Free PMC article.
-
Synaptopodin Regulates Denervation-Induced Plasticity at Hippocampal Mossy Fiber Synapses.Cells. 2024 Jan 6;13(2):114. doi: 10.3390/cells13020114. Cells. 2024. PMID: 38247806 Free PMC article.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. - PubMed
-
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. - PubMed
-
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. - PubMed
-
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
