Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways
- PMID: 17898229
- PMCID: PMC6673141
- DOI: 10.1523/JNEUROSCI.2444-07.2007
Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways
Abstract
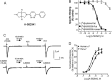
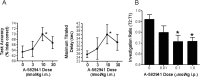
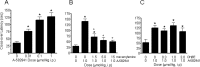
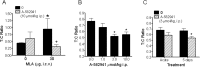
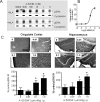
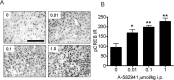
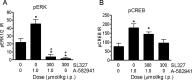
The alpha7 nicotinic acetylcholine receptor (nAChR) plays an important role in cognitive processes and may represent a drug target for treating cognitive deficits in neurodegenerative and psychiatric disorders. In the present study, we used a novel alpha7 nAChR-selective agonist, 2-methyl-5-(6-phenyl-pyridazin-3-yl)-octahydro-pyrrolo[3,4-c]pyrrole (A-582941) to interrogate cognitive efficacy, as well as examine potential cellular mechanisms of cognition. Exhibiting high affinity to native rat (Ki = 10.8 nM) and human (Ki = 16.7 nM) alpha7 nAChRs, A-582941 enhanced cognitive performance in behavioral assays including the monkey delayed matching-to-sample, rat social recognition, and mouse inhibitory avoidance models that capture domains of working memory, short-term recognition memory, and long-term memory consolidation, respectively. In addition, A-582941 normalized sensory gating deficits induced by the alpha7 nAChR antagonist methyllycaconitine in rats, and in DBA/2 mice that exhibit a natural sensory gating deficit. Examination of signaling pathways known to be involved in cognitive function revealed that alpha7 nAChR agonism increased extracellular-signal regulated kinase 1/2 (ERK1/2) phosphorylation in PC12 cells. Furthermore, increases in ERK1/2 and cAMP response element-binding protein (CREB) phosphorylation were observed in mouse cingulate cortex and/or hippocampus after acute A-582941 administration producing plasma concentrations in the range of alpha7 binding affinities and behavioral efficacious doses. The MEK inhibitor SL327 completely blocked alpha7 agonist-evoked ERK1/2 phosphorylation. Our results demonstrate that alpha7 nAChR agonism can lead to broad-spectrum efficacy in animal models at doses that enhance ERK1/2 and CREB phosphorylation/activation and may represent a mechanism that offers potential to improve cognitive deficits associated with neurodegenerative and psychiatric diseases, such as Alzheimer's disease and schizophrenia.
Figures
References
-
- Adams CE, Stitzel JA, Collins AC, Freedman R. Alpha7-nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res. 2001;922:180–190. - PubMed
-
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
-
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. - PubMed
-
- Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–523. - PubMed
-
- Biton B, Bergis OE, Galli F, Nedelec A, Lochead AW, Jegham S, Godet D, Lanneau C, Santamaria R, Chesney F, Leonardon J, Granger P, Debono MW, Bohme GA, Sgard F, Besnard F, Graham D, Coste A, Oblin A, Curet O, et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
