SOD2 knockdown mouse model of early AMD
- PMID: 17898259
- PMCID: PMC6549721
- DOI: 10.1167/iovs.07-0432
SOD2 knockdown mouse model of early AMD
Abstract
Purpose: To test the hypothesis that oxidative injury to the retinal pigment epithelium (RPE) may lead to retinal damage similar to that associated with the early stages of age-related macular degeneration (AMD).
Methods: A ribozyme that targets the protective enzyme manganese superoxide dismutase (MnSOD) was expressed in RPE-J cells, and adeno-associated virus (AAV) expressing the ribozyme gene was injected beneath the retinas of adult C57BL/6 mice. The RPE/choroid complex was examined for SOD2 protein levels and protein markers of oxidative damage using immunoblot analysis and LC MS/MS-identification of proteins and nitration sites. Lipids were extracted from retinal tissue and analyzed for the bis-retinoid compounds A2E and iso-A2E. The mice were analyzed by full-field electroretinography (ERG) for light response. Light and electron microscopy were used to measure cytological changes in the retinas.
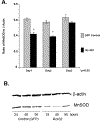
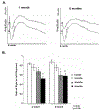
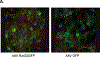
Results: The treatment of RPE-J cells with Rz432 resulted in decreased MnSOD mRNA and protein as well as increased levels of superoxide anion and apoptotic cell death. When delivered by AAV, Rz432 reduced MnSOD protein and increased markers of oxidative damage, including nitrated and carboxyethylpyrrole-modified proteins in the RPE-choroid of mice. Ribozyme delivery caused a progressive loss of electroretinograph response, vacuolization, degeneration of the RPE, thickening of Bruch's membrane, and shortening and disorganization of the photoreceptor outer and inner segments. Progressive thinning of the photoreceptor outer nuclear layer resulted from apoptotic cell death. Similar to the eyes of patients with AMD, ribozyme-treated eyes exhibited increased autofluorescence and elevated levels of A2E and iso-A2E, major bis-retinoid pigments of lipofuscin.
Conclusions: These results support the hypothesis that oxidative damage to the RPE may play a role in some of the key features of AMD.
Figures









References
-
- Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. - PubMed
-
- Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24:335–610. - PubMed
-
- Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-relatedmaculopathy in Australia: the Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–1460. - PubMed
-
- Vingerling JR, Dielemans I, Hofman A, et al. The prevalence ofage-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205–210. - PubMed
-
- Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987;31:291–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY08571/EY/NEI NIH HHS/United States
- EY13729/EY/NEI NIH HHS/United States
- EY15638/EY/NEI NIH HHS/United States
- R01 EY016073/EY/NEI NIH HHS/United States
- P01 NS036302/NS/NINDS NIH HHS/United States
- R01 EY014239/EY/NEI NIH HHS/United States
- NS36302/NS/NINDS NIH HHS/United States
- EY12951/EY/NEI NIH HHS/United States
- EY11123/EY/NEI NIH HHS/United States
- U10 EY013729/EY/NEI NIH HHS/United States
- P30 EY008571/EY/NEI NIH HHS/United States
- EY14239/EY/NEI NIH HHS/United States
- R01 EY020825/EY/NEI NIH HHS/United States
- R01 EY012951/EY/NEI NIH HHS/United States
- R24 EY015638/EY/NEI NIH HHS/United States
- EY016073/EY/NEI NIH HHS/United States
- R01 EY011123/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

