The type I fatty acid and polyketide synthases: a tale of two megasynthases
- PMID: 17898897
- PMCID: PMC2263081
- DOI: 10.1039/b603600g
The type I fatty acid and polyketide synthases: a tale of two megasynthases
Abstract
This review chronicles the synergistic growth of the fields of fatty acid and polyketide synthesis over the last century. In both animal fatty acid synthases and modular polyketide synthases, similar catalytic elements are covalently linked in the same order in megasynthases. Whereas in fatty acid synthases the basic elements of the design remain immutable, guaranteeing the faithful production of saturated fatty acids, in the modular polyketide synthases, the potential of the basic design has been exploited to the full for the elaboration of a wide range of secondary metabolites of extraordinary structural diversity.
Figures







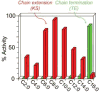


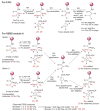

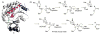
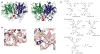


References
-
- Staunton J, Weissman KJ. Nat Prod Rep. 2001;18:380. - PubMed
-
- Shen B. Curr Opin Chem Biol. 2003;7:285. - PubMed
-
- Taylor WC. Snows of yesteryear: J Norman Collie, mountaineer. Holt, Rinehart and Winston; Toronto: 1973.
-
- Collie J. J Chem Soc, Trans. 1907;91:1806.
-
- Raper H. J Chem Soc, Trans. 1907;91:1831.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

