Leaf-associated bacterial and fungal taxa shifts in response to larvae of the tree hole mosquito, Ochlerotatus triseriatus
- PMID: 17899246
- PMCID: PMC4053173
- DOI: 10.1007/s00248-007-9310-6
Leaf-associated bacterial and fungal taxa shifts in response to larvae of the tree hole mosquito, Ochlerotatus triseriatus
Abstract
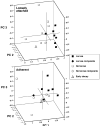
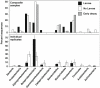
Larvae of the eastern tree hole mosquito, Ochlerotatus triseriatus (Say), and related container-breeding species are known to feed upon substrate-associated microorganisms. Although the importance of these microbial resources to larval growth has been established, almost nothing is known about the taxonomic composition and dynamics of these critical microbial food sources. We examined bacterial and fungal community compositional changes on oak leaves tethered in natural tree hole habitats of O. triseriatus. We eliminated larvae experimentally in a subset of the tree holes and examined 16S rDNA gene sequences for bacteria and ergosterol concentrations and 18S rRNA gene sequences for fungi collected from leaf material subsamples. Leaf ergosterol content varied significantly with time, but not treatment. Principal component analysis (PCA) was used to compare microbial taxonomic patterns found in leaves incubated with or without larvae present, and we found that larval presence affected both bacterial and fungal groups, either from loosely attached or strongly adherent categories. Bacterial communities generally grouped more tightly when larvae were present, and class level taxa proportions changed when larvae were present, suggesting selection by larval feeding or activities for particular taxa such as members of the Bacteroidetes, Alphaproteobacteria, and Betaproteobacteria classes. Fungal taxa composite scores also separated along PC axes related to the presence of larvae and indicated larval feeding effects on several higher taxonomic groups, including Saccharomycetes, Dothideomycetes, and Chytridiomycota. These results support the hypothesis that larval mosquito feeding and activities altered microbial communities associated with substrate surfaces, potentially leading to decreased food value of the resource and affecting decomposition of particulate matter in the system.
Figures





Similar articles
-
Indirect effects of soluble nitrogen on growth of Ochlerotatus triseriatus larvae in container habitats.J Med Entomol. 2006 Jul;43(4):677-88. doi: 10.1603/0022-2585(2006)43[677:ieosno]2.0.co;2. J Med Entomol. 2006. PMID: 16892624
-
Bacterial community structure in tree hole habitats of Ochlerotatus triseriatus: influences of larval feeding.J Am Mosq Control Assoc. 2008 Jun;24(2):219-27. doi: 10.2987/5666.1. J Am Mosq Control Assoc. 2008. PMID: 18666529 Free PMC article.
-
Beetle (Coleoptera: Scirtidae) facilitation of larval mosquito growth in tree hole habitats is linked to multitrophic microbial interactions.Microb Ecol. 2011 Oct;62(3):690-703. doi: 10.1007/s00248-011-9872-1. Epub 2011 May 24. Microb Ecol. 2011. PMID: 21607876 Free PMC article.
-
Effects of larval mosquitoes (Aedes triseriatus) and stemflow on microbial community dynamics in container habitats.Appl Environ Microbiol. 1999 Jun;65(6):2661-73. doi: 10.1128/AEM.65.6.2661-2673.1999. Appl Environ Microbiol. 1999. PMID: 10347058 Free PMC article.
-
Alteration of microbial communities colonizing leaf litter in a temperate woodland stream by growth of trees under conditions of elevated atmospheric CO2.Appl Environ Microbiol. 2010 Aug;76(15):4950-9. doi: 10.1128/AEM.00221-10. Epub 2010 Jun 11. Appl Environ Microbiol. 2010. PMID: 20543045 Free PMC article.
Cited by
-
Ubiquitous water-soluble molecules in aquatic plant exudates determine specific insect attraction.PLoS One. 2008 Oct 8;3(10):e3350. doi: 10.1371/journal.pone.0003350. PLoS One. 2008. PMID: 18841203 Free PMC article.
-
Density-dependence and different dimensions of changing weather shape adult abundance patterns of common mosquito species (Diptera: Culicidae) in Bloomington, Indiana, USA.Curr Res Parasitol Vector Borne Dis. 2025 Jan 7;7:100242. doi: 10.1016/j.crpvbd.2025.100242. eCollection 2025. Curr Res Parasitol Vector Borne Dis. 2025. PMID: 39968057 Free PMC article.
-
Effect of leaf type and pesticide exposure on abundance of bacterial taxa in mosquito larval habitats.PLoS One. 2013 Aug 5;8(8):e71812. doi: 10.1371/journal.pone.0071812. Print 2013. PLoS One. 2013. PMID: 23940789 Free PMC article.
-
Plant compartment and genetic variation drive microbiome composition in switchgrass roots.Environ Microbiol Rep. 2019 Apr;11(2):185-195. doi: 10.1111/1758-2229.12727. Epub 2019 Jan 31. Environ Microbiol Rep. 2019. PMID: 30537406 Free PMC article.
-
Developmental succession of the microbiome of Culex mosquitoes.BMC Microbiol. 2015 Jul 24;15:140. doi: 10.1186/s12866-015-0475-8. BMC Microbiol. 2015. PMID: 26205080 Free PMC article.
References
-
- Alexopoulous CJ, Mims CW, Blackwell M. Introductory Mycology. John Wiley & Sons; New York: 1996. Phylum Chytridiomycota; pp. 86–126.
-
- Amann R, Ludwig W. Ribosomal RNA-targeted nucleic acid probes for studies in microbial ecology. FEMS Microbiol Rev. 2000;24:555–565. - PubMed
-
- Bell T, Ager D, Song JI, Newman JA, Thompson IP, Lilley AK, van der Gast CJ. Larger islands house more bacterial taxa. Science. 2005;308:1884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

