Intraperitoneal photodynamic therapy for an ovarian cancer ascite model in Fischer 344 rat using hematoporphyrin monomethyl ether
- PMID: 17900310
- PMCID: PMC11158014
- DOI: 10.1111/j.1349-7006.2007.00628.x
Intraperitoneal photodynamic therapy for an ovarian cancer ascite model in Fischer 344 rat using hematoporphyrin monomethyl ether
Abstract
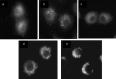
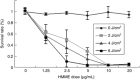
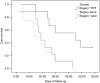
With limited treatment options, intraperitoneal spread of ovarian cancer is a common problem leading to high morbidity. Intraperitoneal photodynamic therapy combined with debulking surgery to treat residual disease is an alternative choice for clinicians. Hematoporphyrin monomethyl ether (HMME) is a promising second-generation photosensitizer developed in China. Our study was designed to investigate the phototoxicity of HMME on ovarian cancer. NuTu-19, a cell line derived from adenocarcinoma of Fischer 344 rat, and its allogeneic graft ascites tumor model was used in this study. HMME was confirmed to be localized in cytolysosome, and HMME-based photosensitization induced direct necrosis as well as mitochondria damage. The photocytotoxicity of HMME was both light- and drug dose-dependent and no significant dark cytotoxicity was observed in NuTu-19 cells. With the ascite tumor-bearing Fischer 344 rat model, HMME-based intraperitoneal photodynamic therapy was proved to be useful in improving the prognosis of ovarian cancer. Thus, this study provides evidence that HMME-based photodynamic therapy is an effective adjuvant therapy for ovarian cancer.
Figures


Similar articles
-
Effect of hematoporphyrin monomethyl ether-mediated PDT on the mitochondria of canine breast cancer cells.Photodiagnosis Photodyn Ther. 2013 Dec;10(4):414-21. doi: 10.1016/j.pdpdt.2013.03.005. Epub 2013 Apr 25. Photodiagnosis Photodyn Ther. 2013. PMID: 24284094
-
Effects of photodynamic therapy using hematoporphyrin monomethyl ether on experimental choroidal neovascularization.Photochem Photobiol. 2010 Jul-Aug;86(4):972-80. doi: 10.1111/j.1751-1097.2010.00757.x. Epub 2010 Jun 10. Photochem Photobiol. 2010. PMID: 20553408
-
[Photodynamic effect of hematoporphyrin monomethyl ether on ovarian cancer cell line SKOV3].Ai Zheng. 2006 Sep;25(9):1108-12. Ai Zheng. 2006. PMID: 16965651 Chinese.
-
Recent progress in hematoporphyrin monomethyl ether-photodynamic therapy for port-wine stains: updates and insights.Arch Dermatol Res. 2024 Nov 16;317(1):28. doi: 10.1007/s00403-024-03531-x. Arch Dermatol Res. 2024. PMID: 39549139 Review.
-
Clinical study on clinical operation and post-treatment reactions of HMME-PDT in treatment of PWS.Photodiagnosis Photodyn Ther. 2017 Dec;20:253-256. doi: 10.1016/j.pdpdt.2017.09.013. Epub 2017 Oct 24. Photodiagnosis Photodyn Ther. 2017. PMID: 29079350 Review.
Cited by
-
Photodynamic therapy for cancer: Role of natural products.Photodiagnosis Photodyn Ther. 2019 Jun;26:395-404. doi: 10.1016/j.pdpdt.2019.04.033. Epub 2019 May 4. Photodiagnosis Photodyn Ther. 2019. PMID: 31063860 Free PMC article. Review.
-
Potential role of organic anion transporting polypeptide 1B1 (OATP1B1) in the selective hepatic uptake of hematoporphyrin monomethyl ether isomers.Acta Pharmacol Sin. 2015 Feb;36(2):268-80. doi: 10.1038/aps.2014.104. Epub 2014 Nov 24. Acta Pharmacol Sin. 2015. PMID: 25418376 Free PMC article.
-
Impact of treatment response metrics on photodynamic therapy planning and outcomes in a three-dimensional model of ovarian cancer.J Biomed Opt. 2013 Sep;18(9):098004. doi: 10.1117/1.JBO.18.9.098004. J Biomed Opt. 2013. PMID: 24802230 Free PMC article.
-
Photochemical Targeting of Mitochondria to Overcome Chemoresistance in Ovarian Cancer †.Photochem Photobiol. 2023 Mar;99(2):448-468. doi: 10.1111/php.13723. Epub 2022 Oct 19. Photochem Photobiol. 2023. PMID: 36117466 Free PMC article. Review.
-
Recent progress in sono-photodynamic cancer therapy: From developed new sensitizers to nanotechnology-based efficacy-enhancing strategies.Acta Pharm Sin B. 2021 Aug;11(8):2197-2219. doi: 10.1016/j.apsb.2020.12.016. Epub 2020 Dec 21. Acta Pharm Sin B. 2021. PMID: 34522584 Free PMC article. Review.
References
-
- American Cancer Society . Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society, 2007.
-
- Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B 1997; 39: 1–18. - PubMed
-
- Triesscheijn M, Baas P, Schellens JH et al . Photodynamic therapy in oncology. Oncologist 2006; 11: 1034–44. - PubMed
-
- Tochner Z, Mitchell JB, Harrington FS et al . Treatment of murine intraperitoneal ovarian ascitic tumor with hematoporphyrin derivative and laser light. Cancer Res 1985; 45: 2983–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

