Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain
- PMID: 17900333
- PMCID: PMC2093929
- DOI: 10.1186/1744-8069-3-27
Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain
Abstract
Background: Disinhibition of neurons in the superficial spinal dorsal horn, via microglia - neuron signaling leading to disruption of chloride homeostasis, is a potential cellular substrate for neuropathic pain. But, a central unresolved question is whether this disinhibition can transform the activity and responses of spinal nociceptive output neurons to account for the symptoms of neuropathic pain.
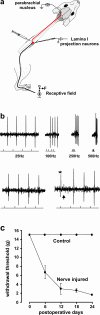
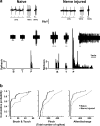
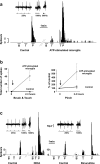
Results: Here we show that peripheral nerve injury, local spinal administration of ATP-stimulated microglia or pharmacological disruption of chloride transport change the phenotype of spinal lamina I output neurons, causing them to 1) increase the gain of nociceptive responsiveness, 2) relay innocuous mechanical input and 3) generate spontaneous bursts of activity. The changes in the electrophysiological phenotype of lamina I neurons may account for three principal components of neuropathic pain: hyperalgesia, mechanical allodynia and spontaneous pain, respectively.
Conclusion: The transformation of discharge activity and sensory specificity provides an aberrant signal in a primarily nociceptive ascending pathway that may serve as a basis for the symptoms of neuropathic pain.
Figures

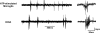
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

