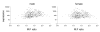Regional differences in dosage compensation on the chicken Z chromosome
- PMID: 17900367
- PMCID: PMC2375040
- DOI: 10.1186/gb-2007-8-9-r202
Regional differences in dosage compensation on the chicken Z chromosome
Abstract
Background: Most Z chromosome genes in birds are expressed at a higher level in ZZ males than in ZW females, and thus are relatively ineffectively dosage compensated. Some Z genes are compensated, however, by an unknown mechanism. Previous studies identified a non-coding RNA in the male hypermethylated (MHM) region, associated with sex-specific histone acetylation, which has been proposed to be involved in dosage compensation.
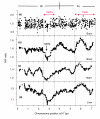
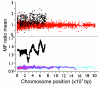
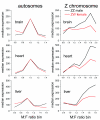
Results: Using microarray mRNA expression analysis, we find that dosage compensated and non-compensated genes occur across the Z chromosome, but a cluster of compensated genes are found in the MHM region of chicken chromosome Zp, whereas Zq is enriched in non-compensated genes. The degree of dosage compensation among Z genes is predicted better by the level of expression of Z genes in males than in females, probably because of better compensation of genes with lower levels of expression. Compensated genes have different functional properties than non-compensated genes, suggesting that dosage compensation has evolved gene-by-gene according to selective pressures on each gene. The group of genes comprising the MHM region also resides on a primitive mammalian (platypus) sex chromosome and, thus, may represent an ancestral precursor to avian ZZ/ZW and monotreme XX/XY sex chromosome systems.
Conclusion: The aggregation of dosage compensated genes near the MHM locus may reflect a local sex- and chromosome-specific mechanism of dosage compensation, perhaps mediated by the MHM non-coding RNA.
Figures