Inflammatory conditions induce gap junctional communication between rat Kupffer cells both in vivo and in vitro
- PMID: 17900549
- PMCID: PMC2105790
- DOI: 10.1016/j.cellimm.2007.08.001
Inflammatory conditions induce gap junctional communication between rat Kupffer cells both in vivo and in vitro
Abstract
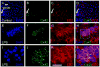
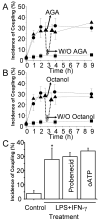
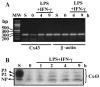
Connexin43 (Cx43), a gap junction protein subunit, has been previously detected in Kupffer cells (KCs) during liver inflammation, however, KCs phagocytose cell debris that may include Cx43 protein, which could explain the detection of Cx43 in KCs. We determined that KCs express Cx43 and form gap junctions (GJs) both in vivo and in vitro. In liver sections of animals treated with LPS, Cx43 was detected at ED2+ cells interfaces, indicating formation of GJs between KCs in vivo. In vitro, unstimulated KCs cultures did not form functional GJs, and expressed low levels of Cx43 that showed a diffuse intracellular distribution. In contrast, KCs treated with LPS plus IFN-gamma, expressed a greater amount of Cx43 at both, protein and mRNA levels, and showed Cx43 at cell-cell contacts associated with higher dye coupling. In conclusion, activation of KCs in vivo or in vitro resulted in enhanced Cx43 expression levels and formation of GJ that might play relevant roles during liver inflammation.
Figures

Similar articles
-
Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha.Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):4190-5. doi: 10.1073/pnas.051634298. Epub 2001 Mar 20. Proc Natl Acad Sci U S A. 2001. PMID: 11259646 Free PMC article.
-
Gap junctional communication promotes apoptosis in a connexin-type-dependent manner.Cell Death Dis. 2013 Apr 11;4(4):e584. doi: 10.1038/cddis.2013.105. Cell Death Dis. 2013. PMID: 23579271 Free PMC article.
-
TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses.J Immunol. 2003 Feb 1;170(3):1320-8. doi: 10.4049/jimmunol.170.3.1320. J Immunol. 2003. PMID: 12538692
-
Astrocytic Connexin43 Channels as Candidate Targets in Epilepsy Treatment.Biomolecules. 2020 Nov 20;10(11):1578. doi: 10.3390/biom10111578. Biomolecules. 2020. PMID: 33233647 Free PMC article. Review.
-
Biological Functions of Connexin43 Beyond Intercellular Communication.Trends Cell Biol. 2019 Oct;29(10):835-847. doi: 10.1016/j.tcb.2019.07.001. Epub 2019 Jul 26. Trends Cell Biol. 2019. PMID: 31358412 Review.
Cited by
-
Distinct behavior of myelomonocytic cells and CD8 T cells underlies the hepatic response to Listeria monocytogenes.Wellcome Open Res. 2018 Apr 24;3:48. doi: 10.12688/wellcomeopenres.12941.1. eCollection 2018. Wellcome Open Res. 2018. PMID: 29862325 Free PMC article.
-
Modulation of connexin signaling by bacterial pathogens and their toxins.Cell Mol Life Sci. 2011 Sep;68(18):3047-64. doi: 10.1007/s00018-011-0737-z. Epub 2011 Jun 9. Cell Mol Life Sci. 2011. PMID: 21656255 Free PMC article. Review.
-
Involvement of connexin43 in acetaminophen-induced liver injury.Biochim Biophys Acta. 2016 Jun;1862(6):1111-21. doi: 10.1016/j.bbadis.2016.02.007. Epub 2016 Feb 18. Biochim Biophys Acta. 2016. PMID: 26912412 Free PMC article.
-
Connexins and their channels in inflammation.Crit Rev Biochem Mol Biol. 2016 Nov/Dec;51(6):413-439. doi: 10.1080/10409238.2016.1204980. Epub 2016 Jul 7. Crit Rev Biochem Mol Biol. 2016. PMID: 27387655 Free PMC article. Review.
-
Connexin-based signaling and drug-induced hepatotoxicity.J Clin Transl Res. 2017 Feb;3(Suppl 1):189-198. doi: 10.18053/jctres.03.2017S1.004. Epub 2017 Feb 12. J Clin Transl Res. 2017. PMID: 28825041 Free PMC article.
References
-
- Racanelli V, Rehermann B. Hepatology. 2006;43:S54–62. - PubMed
-
- Springer TA. Nature. 1990;346:425–34. - PubMed
-
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Physiol Rev. 2003;83:1359–400. - PubMed
-
- MacPhee PJ, Schmidt EE, Groom AC. Am J Physiol. 1992;263:G17–23. - PubMed
-
- Gonzalez HE, Eugenin EA, Garces G, Solis N, Pizarro M, Accatino L, Saez JC. Am J Physiol Gastrointest Liver Physiol. 2002;282:G991–G1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

