Kinetic analysis of the slow skeletal myosin MHC-1 isoform from bovine masseter muscle
- PMID: 17900618
- PMCID: PMC2098880
- DOI: 10.1016/j.jmb.2007.08.050
Kinetic analysis of the slow skeletal myosin MHC-1 isoform from bovine masseter muscle
Abstract
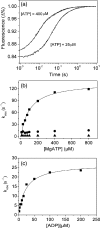
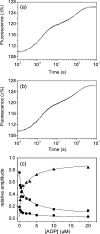
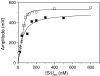
Several heavy chain isoforms of class II myosins are found in muscle fibres and show a large variety of different mechanical activities. Fast myosins (myosin heavy chain (MHC)-II-2) contract at higher velocities than slow myosins (MHC-II-1, also known as beta-myosin) and it has been well established that ADP binding to actomyosin is much tighter for MHC-II-1 than for MHC-II-2. Recently, we reported several other differences between MHC-II isoforms 1 and 2 of the rabbit. Isoform II-1 unlike II-2 gave biphasic dissociation of actomyosin by ATP, the ATP-cleavage step was significantly slower for MHC-II-1 and the slow isoforms showed the presence of multiple actomyosin-ADP complexes. These results are in contrast to published data on MHC-II-1 from bovine left ventricle muscle, which was more similar to the fast skeletal isoform. Bovine MHC-II-1 is the predominant isoform expressed in both the ventricular myocardium and slow skeletal muscle fibres such as the masseter and is an important source of reference work for cardiac muscle physiology. This work examines and extends the kinetics of bovine MHC-II-1. We confirm the primary findings from the work on rabbit soleus MHC-II-1. Of significance is that we show that the affinity of ADP for bovine masseter myosin in the absence of actin (represented by the dissociation constant K(D)) is weaker than originally described for bovine cardiac myosin and thus the thermodynamic coupling between ADP and actin binding to myosin is much smaller (K(AD)/K(D) approximately 5 instead of K(AD)/K(D) approximately 50). This may indicate a distinct type of mechanochemical coupling for this group of myosin motors. We also find that the ATP-hydrolysis rate is much slower for bovine MHC-II-1 (19 s(-1)) than reported previously (138 s(-1)). We discuss how this work fits into a broader characterisation of myosin motors from across the myosin family.
Figures







References
-
- De La Cruz E.M., Ostap E.M. Relating biochemistry and function in the myosin superfamily. Curr. Opin. Cell Biol. 2004;16:61–67. - PubMed
-
- Geeves M.A., Holmes K.C. The molecular mechanism of muscle contraction. Adv. Protein Chem. 2005;71:161–193. - PubMed
-
- Reggiani C., Bottinelli R., Stienen G.J. Sarcomeric myosin isoforms: fine tuning of a molecular motor. News Physiol. Sci. 2000;15:26–33. - PubMed
-
- Timson D.J. Fine tuning the myosin motor: the role of the essential light chain in striated muscle myosin. Biochimie. 2003;85:639–645. - PubMed
-
- Schiaffino S., Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol. Rev. 1996;76:371–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

