Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation
- PMID: 17901071
- PMCID: PMC2441762
- DOI: 10.1095/biolreprod.107.064477
Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation
Abstract
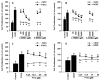
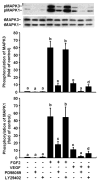
Fibroblast growth factor-2 (FGF2) and vascular endothelial growth factor (VEGF) are two key regulators of placental angiogenesis. The potent vasodilator nitric oxide (NO) could also act as a key mediator of FGF2- and VEGF-induced angiogenesis. However, the postreceptor signaling pathways governing these FGF2- and VEGF-induced placental angiogenic responses are poorly understood. In this study, we assessed the role of endogenous NO, mitogen-activated protein kinase 3/1 (MAPK3/1), and v-akt murine thymoma viral oncogene homolog 1 (AKT1) in FGF2- and VEGF-stimulated proliferation of ovine fetoplacental endothelial (OFPAE) cells. Both FGF2 and VEGF time-dependently stimulated (P < 0.05) NO production and activated AKT1. Both FGF2- and VEGF-stimulated cell proliferation was dose-dependently inhibited (P < 0.05) by N(G)-monomethyl-L-arginine (L-NMMA; an NO synthase inhibitor), PD98059 (a selective MAPK3/1 kinase 1 and 2 [MAP2K1/2] inhibitor), or LY294002 (a selective phosphatidylinositol 3 kinase [PI3K] inhibitor) but not by phenyl-4,4,5,5 tetramethylimidazoline-1-oxyl 3-oxide (PTIO, a potent extracellular NO scavenger). At the maximal inhibitory dose without cytotoxicity, PD98059 and LY294002 completely inhibited VEGF-induced cell proliferation but only partially attenuated (P < 0.05) FGF2-induced cell proliferation. PD98059 and LY294002 also inhibited (P < 0.05) FGF2- and VEGF-induced phosphorylation of MAPK3/1 and AKT1, respectively. L-NMMA did not significantly affect FGF2- and VEGF-induced phosphorylation of either MAPK3/1 or AKT1. Thus, in OFPAE cells, both FGF2- and VEGF-stimulated cell proliferation is partly mediated via NO as an intracellular and downstream signal of MAPK3/1 and AKT1 activation. Moreover, activation of both MAP2K1/2/MAPK3/1 and PI3K/AKT1 pathways is critical for FGF2-stimulated cell proliferation, whereas activation of either one pathway is sufficient for mediating the VEGF-induced maximal cell proliferation, indicating that these two kinase pathways differentially mediate the FGF2- and VEGF-stimulated OFPAE cell proliferation.
Figures







Similar articles
-
Protein phosphatase 3 differentially modulates vascular endothelial growth factor- and fibroblast growth factor 2-stimulated cell proliferation and signaling in ovine fetoplacental artery endothelial cells.Biol Reprod. 2008 Oct;79(4):704-10. doi: 10.1095/biolreprod.108.068957. Epub 2008 May 28. Biol Reprod. 2008. PMID: 18509162 Free PMC article.
-
Hypoxia enhances FGF2- and VEGF-stimulated human placental artery endothelial cell proliferation: roles of MEK1/2/ERK1/2 and PI3K/AKT1 pathways.Placenta. 2009 Dec;30(12):1045-51. doi: 10.1016/j.placenta.2009.10.007. Epub 2009 Nov 5. Placenta. 2009. PMID: 19892399 Free PMC article.
-
Exogenous nitric oxide stimulates cell proliferation via activation of a mitogen-activated protein kinase pathway in ovine fetoplacental artery endothelial cells.Biol Reprod. 2006 Feb;74(2):375-82. doi: 10.1095/biolreprod.105.043190. Epub 2005 Oct 26. Biol Reprod. 2006. PMID: 16251502
-
Suppression of protein phosphatase 2 differentially modulates VEGF- and FGF2-induced signaling in ovine fetoplacental artery endothelial cells.Placenta. 2009 Oct;30(10):907-13. doi: 10.1016/j.placenta.2009.07.003. Epub 2009 Aug 18. Placenta. 2009. PMID: 19692121 Free PMC article.
-
Signaling regulation of fetoplacental angiogenesis.J Endocrinol. 2012 Mar;212(3):243-55. doi: 10.1530/JOE-11-0296. Epub 2011 Nov 21. J Endocrinol. 2012. PMID: 22106098 Free PMC article. Review.
Cited by
-
Placental surface area mediates the association between FGFR2 methylation in placenta and full-term low birth weight in girls.Clin Epigenetics. 2018 Mar 22;10:39. doi: 10.1186/s13148-018-0472-5. eCollection 2018. Clin Epigenetics. 2018. PMID: 29588807 Free PMC article.
-
Therapeutic effects of a liquid bandage prepared with cellulose powders from Styela clava tunics and Broussonetia kazinoki bark: Healing of surgical wounds on the skin of Sprague Dawley rats.Mol Med Rep. 2019 Jan;19(1):452-460. doi: 10.3892/mmr.2018.9668. Epub 2018 Nov 19. Mol Med Rep. 2019. PMID: 30483728 Free PMC article.
-
Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae.J Cell Physiol. 2012 Jun;227(6):2480-91. doi: 10.1002/jcp.22984. J Cell Physiol. 2012. PMID: 21830216 Free PMC article.
-
Activation of AP-1 transcription factors differentiates FGF2 and vascular endothelial growth factor regulation of endothelial nitric-oxide synthase expression in placental artery endothelial cells.J Biol Chem. 2010 Jun 4;285(23):17348-58. doi: 10.1074/jbc.M109.092791. Epub 2010 Apr 6. J Biol Chem. 2010. PMID: 20371606 Free PMC article.
-
Extracellular Vesicles and Their miRNA Content in Amniotic and Tracheal Fluids of Fetuses with Severe Congenital Diaphragmatic Hernia Undergoing Fetal Intervention.Cells. 2021 Jun 14;10(6):1493. doi: 10.3390/cells10061493. Cells. 2021. PMID: 34198576 Free PMC article.
References
-
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–1851. - PubMed
-
- Magness RR, Zheng J. Maternal cardiovascular alterations during pregnancy. In: Gluckman PD, Heymann MA, editors. Pediatric and Perinatal Perspectives: The Scientific Basic. London: Edward Arnold Publishers; 1996. pp. 762–772.
-
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III Changes in complicated pregnancies. Placenta. 2004;25:127–139. - PubMed
-
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

